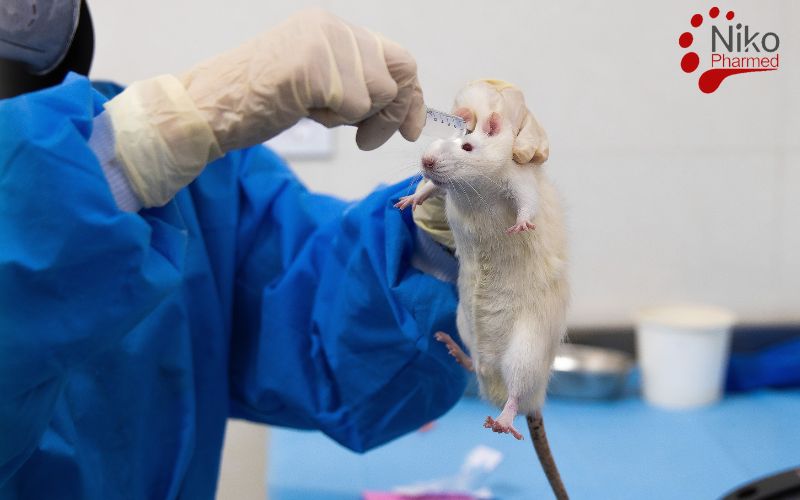
What Is Subacute Systemic Toxicity?
Subacute systemic toxicity, as defined in ISO 10993-11, refers to the adverse effects on a test organism’s internal organs or physiological systems following repeated or continuous exposure to a medical device extract or leachate over a minimum period of 28 days via systemic administration routes (e.g., oral, dermal, subcutaneous, or intravenous). This study aims to evaluate potential cumulative toxic effects resulting from repeated dosing of extractables that may be released from a device during clinical use.
The selection of species (typically rats), dosing regimen, and administration route must reflect the intended clinical exposure and simulate realistic conditions. Key endpoints include clinical signs, body weight trends, hematology, clinical biochemistry, and histopathological evaluation of target organs. According to ISO 10993-11, the extract must be prepared using appropriate vehicles and validated extraction conditions as per ISO 10993-12, ensuring representation of the worst-case clinical exposure scenario. The goal is to ensure that the device does not cause subacute systemic toxicity and thus supports its biological safety profile under extended exposure conditions.
Within the ISO 10993-11 systemic toxicity framework, subacute studies sit between single-dose Acute Systemic Toxicity Testing and longer-term evaluations, and form a core part of the overall Systemic Toxicity Testing strategy for medical devices with repeated exposure.
To request testing or a complimentary consultation contact Nikopharmad
Subacute Systemic Toxicity Testing Procedure
Objective and Scope
The objective of the subacute systemic toxicity study is to evaluate the potential cumulative toxicological effects of leachable substances from a medical device following repeated systemic exposure over a 28-day period, simulating the intended clinical route and duration of use. The study is conducted in accordance with Good Laboratory Practice (GLP) and under the framework of ISO 10993-11, with reference to OECD TG 407 for oral dosing, where applicable.
Selection of Test System
Species: Healthy, young adult Wistar or Sprague-Dawley rats, 6–8 weeks old, typically 200–300g at initiation.
Sex: Both sexes, unless otherwise justified (e.g., sex-specific device use).
Group Size: Minimum 10 animals per sex per group (test and control), to enable statistical power.
Acclimatization: At least 5–7 days prior to dosing under controlled environmental conditions.
Preparation of Test and Control Articles
Extraction Protocol:
Conducted per ISO 10993-12:2021.
Polar and non-polar solvents are selected based on device composition (e.g., physiological saline and cottonseed oil).
Surface area-to-volume ratio follows the standard (typically 6 cm²/mL for polymers).
Extraction conditions: 37°C for 72 hours, unless otherwise justified.
Sterility: Extracts must be sterile filtered and free of particulates or precipitates.
Controls: Use identically prepared vehicle-only solutions for administration to control animals.
Dosing Regimen
Route of Administration: Must reflect the clinical exposure; typically:
Oral gavage for systemic absorption، Intravenous for direct blood exposure (e.g., catheters)
Subcutaneous or intraperitoneal if applicable
Dose Volume:
Oral: Typically 10–20 mL/kg/day
Intravenous: Not to exceed 1–5 mL/kg/day
Frequency and Duration:
Administer once daily for 28 consecutive days
Adjust dose volume weekly based on body weight
In-Life Observations and Monitoring
Clinical Signs:
Observe animals at least once daily, with focused observation after dosing.
Record any signs of toxicity, pain, distress, or abnormal behavior.
Body Weight and Food Consumption:
Record body weight prior to dosing, then weekly.
Monitor food and water consumption at least weekly.
Mortality/Morbidity:
All animals are checked twice daily, weekends included.
Terminal Procedures
Euthanasia:
On Day 29 (±1), animals are humanely euthanized under anesthesia using CO₂ or injectable agents, followed by exsanguination.
Blood Sampling:
Cardiac puncture or vena cava collection for:
Hematology: RBC, WBC, Hb, Hct, platelets, differential count
Clinical Chemistry: ALT, AST, BUN, creatinine, glucose, albumin, electrolytes
Organ Weights:
Record absolute and relative weights of key organs:
Liver, kidneys, spleen, heart, brain, lungs, adrenal glands, testes/ovaries
Necropsy and Histopathology
Gross Necropsy:
Conduct a systematic macroscopic examination of external body and all major organs.
Tissue Collection:
Fix tissues in 10% neutral buffered formalin (or Bouin’s for testes).
Mandatory organs per ISO 10993-11:
Liver, kidneys, spleen, heart, lungs, brain, adrenals, reproductive organs, injection site (if applicable)
Microscopic Examination:
Conducted on all collected tissues for both test and control groups.
Pathological grading (minimal, mild, moderate, severe) by a board-certified toxicologic pathologist.
Data Processing and Interpretation
Statistical Analysis:
Use parametric or non-parametric methods based on data distribution (ANOVA, Dunnett’s, Kruskal-Wallis).
Interpretation:
Evaluate for dose-dependent trends, clinical correlation, and histopathological confirmation.
Consider NOAEL (No Observed Adverse Effect Level) determination if applicable.
Reporting
Compile a comprehensive GLP-compliant report, including:
Justification of animal model and route of administration
Full raw data tables for clinical signs, weights, blood parameters, histopathology
Interpretation of results in the context of device biocompatibility and regulatory safety(Results from subacute toxicity testing are frequently used to refine study design for Subchronic Systemic Toxicity and Chronic Systemic Toxicity studies, helping to select appropriate dose levels, target organs and monitoring plans for extended exposure).
Declaration of study director, QA statement, protocol deviations
Considerations
- Ensure the extract reflects worst-case clinical use scenarios, including potential degradation products.
- Histopathology is critical: absence of clinical signs does not exclude subclinical organ-specific toxicity.
- Control of environmental variables (humidity, temperature, lighting) is essential to reduce confounding.
Subacute Toxicity Testing Lab
At International & Accredited Lab Nikopharmad Laboratory Network, we are committed to providing accurate, reliable, and high-quality biocompatibility testing services for medical devices and pharmaceutical products. Holding the esteemed ISO 17025 certification and ILAC accreditation, we adhere to internationally recognized standards, ensuring that our testing processes meet the highest quality and regulatory requirements.
Certainly. Below is the revised version of the text with a professionally integrated section highlighting competitive pricing, without compromising the formal tone or technical credibility of the message.
Subacute Systemic Toxicity Testing at International Nikopharmad Laboratory
Accreditation and Global Recognition
International Nikopharmad Laboratory is proud to hold ISO/IEC 17025 certification and ILAC accreditation, affirming our compliance with the highest international standards for laboratory competence and quality management. These certifications validate the reliability, traceability, and regulatory acceptance of our test results across global markets, including the U.S. FDA, EU MDR, and other international regulatory bodies.
Specialized Expertise in Subacute Toxicity Testing
As part of our comprehensive biological evaluation services, we offer GLP-compliant Subacute Systemic Toxicity testing in accordance with ISO 10993-11:2017. This critical test assesses the potential cumulative toxicity of medical device extractables when administered systemically over a 28-day period. Our procedures simulate realistic clinical exposures and are designed to detect subclinical and organ-specific toxicological effects through detailed in-life monitoring, hematology, clinical chemistry, necropsy, and histopathology.
Technical Excellence and Advanced Infrastructure
Our laboratory is equipped with state-of-the-art in vivo facilities, precision dosing systems, and validated extraction protocols per ISO 10993-12. Our highly qualified team of toxicologists, pathologists, and GLP specialists ensures the scientific rigor and reproducibility of every study, supporting risk assessment and regulatory submission.
Timely, Accurate, and Regulatory-Ready Results
We recognize that time-to-market is critical in medical device development. Our streamlined workflows and rigorous quality controls enable us to deliver accurate and timely toxicity data, helping you advance through biocompatibility assessments and reach regulatory milestones without delay.
Data Integrity and Confidentiality
Nikopharmad operates under strict confidentiality protocols. All data, samples, and test outcomes are handled in full compliance with your IP protection policies, and our quality system ensures data integrity, traceability, and secure documentation throughout the study lifecycle.
Competitive Pricing Without Compromising Quality
At Nikopharmad, we are committed to offering cost-effective testing solutions without sacrificing quality or compliance. Our competitive pricing model ensures that you receive world-class toxicological evaluations at a cost that supports your R&D budget and accelerates your path to market.
Choose Nikopharmad for Your Subacute Systemic Toxicity Testing
By choosing International Nikopharmad Laboratory, you are partnering with a trusted, accredited facility that not only meets but exceeds international standards for biocompatibility testing. Our dedication to scientific excellence, regulatory compliance, competitive pricing, and customer confidentiality makes us the ideal partner to ensure your medical device or pharmaceutical product is safe, effective, and globally compliant.
To request testing or a complimentary consultation contact Nikopharmad
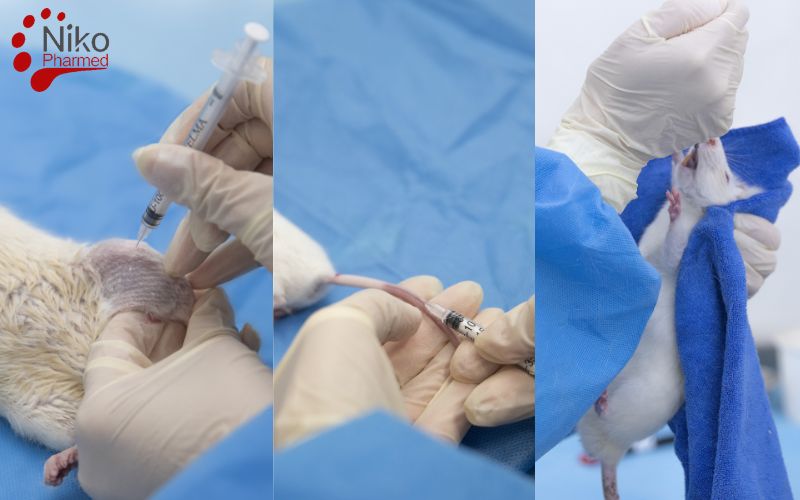
Subacute Systemic Toxicity Testing Administration Routes
In Subacute Systemic Toxicity studies, the route of administration must reflect the intended clinical exposure of the medical device or its extractables. The selection of an appropriate route ensures that the systemic effects observed are relevant and scientifically justified. The following are commonly used routes of administration:
Intraperitoneal (IP)
Intraperitoneal injection delivers the test substance into the peritoneal cavity. It allows for rapid absorption into the systemic circulation and is often used when oral bioavailability is poor. This route is suitable for evaluating systemic effects of materials that might be absorbed from internal applications.
Intramuscular (IM)
Intramuscular administration involves injecting the substance into the skeletal muscle, typically the quadriceps or gluteal muscle. It enables slow and sustained systemic absorption, making it appropriate for evaluating long-term exposure from implanted or injectable materials.
Subcutaneous (SC)
This route involves injection under the skin, providing moderate absorption into systemic circulation. Subcutaneous administration is widely used to simulate systemic exposure from subdermal implants, dermal patches, or injectable formulations.
Gavage (Oral Administration)
Gavage is the direct delivery of the test substance into the stomach using a specialized feeding tube. It is the standard method for assessing systemic toxicity via the oral route, commonly used for devices or materials expected to be ingested or absorbed gastrointestinally.
Intravenous (IV)
Intravenous injection administers the substance directly into the bloodstream, ensuring immediate and complete systemic exposure. It is essential for evaluating the toxicity of intravenous catheters, infusion sets, or parenteral drug-device combinations.
The choice of administration route in Subacute Systemic Toxicity Testing is a critical scientific decision and must be based on the device’s intended clinical use. ISO 10993-11 mandates that the route simulate worst-case systemic exposure to ensure safety and compliance with global biocompatibility standards.
Reference: iso.org