What is Systemic Toxicity Testing?
Systemic toxicity testing evaluates how chemicals, medical devices, or cosmetic ingredients affect the entire body once absorbed into the bloodstream. It identifies potential harmful effects on vital organs such as the liver, kidneys, nervous system, and blood. This testing helps determine safe exposure levels and supports the overall safety assessment of products.
Our systemic toxicity testing services identify potential impacts on vital organs, helping you demonstrate product safety and meet global regulatory requirements. By using reliable, validated methods, we provide clear, actionable data that supports safer product design and faster approvals.
Types of Systemic Toxicity Testing
Acute systemic toxicity
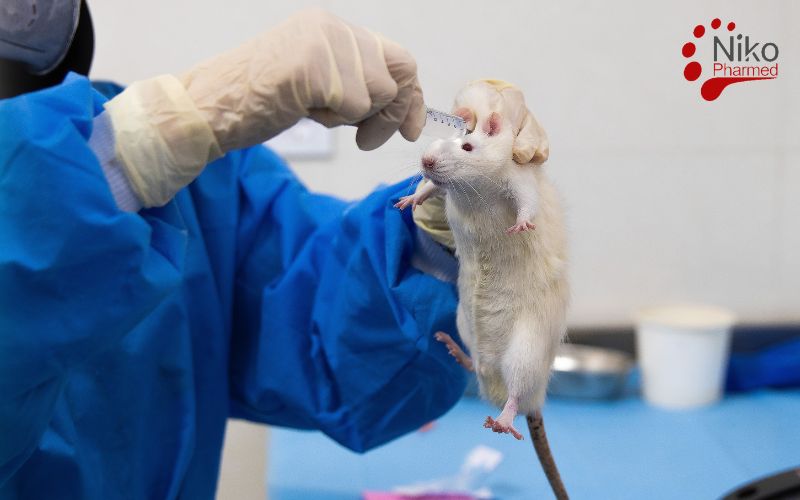
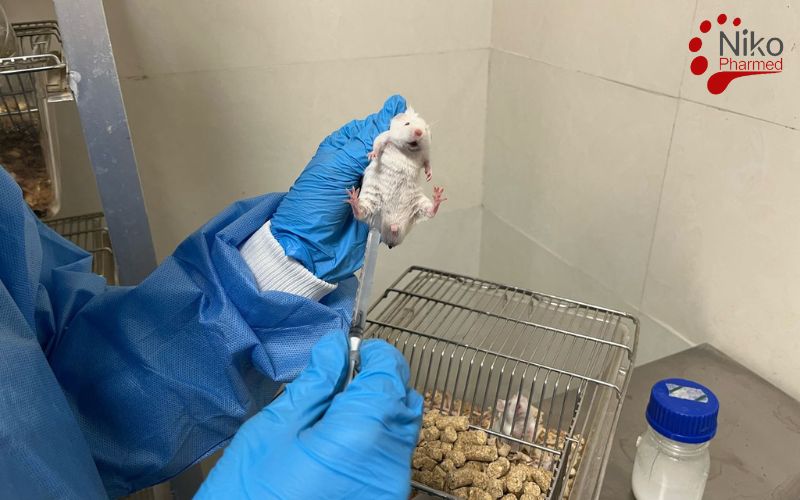
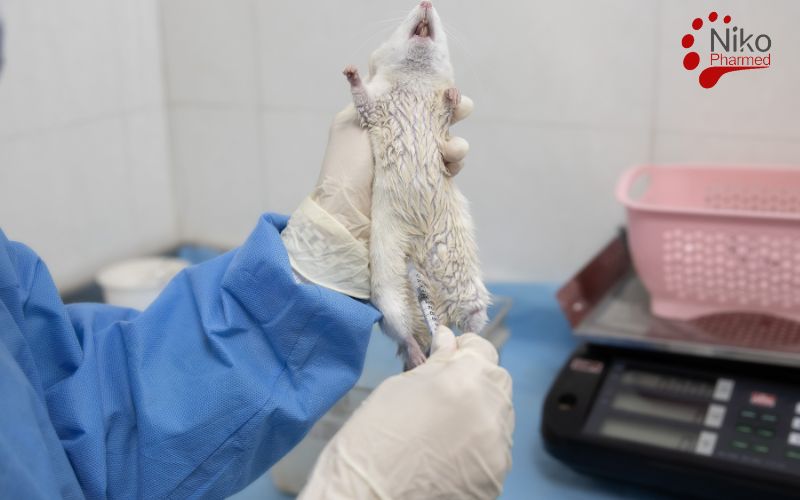
Acute systemic toxicity testing under ISO 10993-11 evaluates the potential adverse effects resulting from a single or limited series of exposures to device extracts within a 24-hour period. In these studies, extracts of the medical device are administered—often intravenously, intraperitoneally, or orally—to a rodent model, and clinical signs such as lethargy, behavioral changes, body-weight loss, and mortality are monitored over 14 days to identify any immediate toxic responses. The primary objective is to define a dose level that does not produce unacceptable systemic effects, thereby establishing a safe exposure threshold for initial clinical use.
Subacute systemic toxicity
Subacute systemic toxicity tests involve repeated or continuous exposure to device extracts for more than 24 hours up to 28 days. During this interval, rodents receive daily administrations of the extract at one or more dose levels, with observations including clinical signs, body-weight trends, food consumption, hematology, clinical chemistry, and gross pathology at study termination. These studies are designed to reveal toxic effects that manifest only after short-term repeated exposures—information critical for devices intended for intermittent use over days to weeks.
Subchronic systemic toxicity
In subchronic systemic toxicity testing, device extracts are administered repeatedly over a portion of the test animal’s lifespan—typically 90 days in rodents but not exceeding 10 % of the species’ normal life span. This medium-term evaluation expands upon subacute studies by assessing cumulative and potentially reversible or progressive toxic effects on organ systems. Endpoints include detailed histopathological examination of major organs (e.g., liver, kidneys, heart, and spleen), alongside more comprehensive clinical pathology panels to ensure detection of any emerging toxicities that could compromise longer-term patient safety.
Chronic systemic toxicity
Chronic systemic toxicity studies extend repeated exposure over a major portion of an animal’s life—generally more than 10 % of its normal life span (e.g., 6–12 months in rodents). These long-term investigations are crucial for implantable or permanent devices, where slow-release or prolonged contact of leachables may induce delayed or progressive organ damage. In addition to all subchronic endpoints, chronic studies monitor survival curves, oncogenic or degenerative lesions, and may include recovery groups to assess reversibility of effects. The comprehensive data support risk assessment for devices intended for lifelong patient use.
Systemic Toxicity Testing Areas
According to ISO 10993-11, systemic toxicity testing of medical-device extracts can be carried out via several administration routes to model different patterns of exposure and absorption:
- Intravenous (IV): delivers the extract directly into the bloodstream, providing immediate systemic exposure and allowing assessment of acute reactions such as anaphylaxis, cardiovascular instability or rapid organ uptake.
- Intraperitoneal (IP): administers the extract into the peritoneal cavity, where it is absorbed through the mesenteric vessels; this route is often used when IV access is challenging, offering rapid but slightly delayed systemic distribution.
- Subcutaneous (SC): deposits the extract beneath the skin, resulting in slower, sustained absorption that mimics devices or coatings releasing leachables into subdermal tissues over time.
- Intramuscular (IM): injects the extract deep into muscle tissue, combining moderate absorption speed with a depot effect, useful for assessing potential myotoxicity as well as systemic responses.
- Oral gavage: delivers the extract directly into the stomach via a feeding tube, modeling ingestion of leachables (e.g., from devices contacting gastrointestinal mucosa) and allowing evaluation of first-pass metabolism and gastrointestinal-mediated toxicity.
By selecting one or more of these routes—alone or in combination—ISO 10993-11 ensures that extracts of a medical device are evaluated under exposure scenarios most relevant to their intended clinical use, thereby revealing potential systemic effects on organs such as liver, kidney, heart or nervous system before human application.
To request testing or a complimentary consultation contact Nikopharmad
What is an example of a systemic effect?
For example, if a novel polymer coating on an implanted vascular stent releases trace chemicals into the bloodstream, those substances may be carried to the liver, where they provoke hepatocellular stress. Clinically, this could manifest as elevated liver enzymes (e.g., ALT, AST) and histopathological signs of mild hepatocellular vacuolation upon biopsy. Such a reaction—remote from the implant site—is a classic systemic toxicity endpoint, demonstrating why ISO 10993-11 mandates in vivo extract testing (e.g., via intravenous or oral administration) to detect potential liver, kidney or cardiovascular impacts before a device enters clinical use.
Importance of Systemic Toxicity Testing
This testing evaluates whether chemicals that leach from a device can produce harmful effects when distributed systemically—via the bloodstream—to distant organs or tissues following exposure.
Technically, acute systemic toxicity tests simulate real-world clinical scenarios to detect early systemic responses, such as neurotoxicity, hepatotoxicity, or cardiovascular effects. These tests are essential for identifying dose-dependent toxicological thresholds and supporting toxicological risk assessments required for regulatory submissions.
this testing has been instrumental in:
- Validating chemical characterization data by linking extractable profiles to observed biological effects.
- Guiding material selection and device design by detecting unintended systemic hazards early in development.
- Supporting ISO 10993-11 compliance, which is mandatory for device types with systemic exposure potential, such as implants or infusion systems.
Systemic Toxicity Testing Lab
At International & Accredited Lab Nikopharmad Laboratory Network, we are committed to providing accurate, reliable, and high-quality Systemic toxicity testing services for medical devices and pharmaceutical products. Holding the esteemed ISO 17025 certification and ILAC accreditation, we adhere to internationally recognized standards, ensuring that our testing processes meet the highest quality and regulatory requirements.
Why Choose Nikopharmad Laboratory?
ILAC accreditation
Nikopharmad Laboratory is proudly certified with ISO 17025 and holds ILAC accreditation. These prestigious certifications ensure that our laboratory operates with the highest standards of quality and competence, providing reliable and internationally recognized testing services for medical devices and pharmaceutical products. Our commitment to maintaining these certifications demonstrates our dedication to excellence and compliance with global regulatory requirements.
Comprehensive Testing Capabilities
We offer a broad range of biocompatibility tests, each designed to assess the safety and performance of your products in compliance with international regulatory frameworks.
Expertise and Experience
With extensive experience in the medical and pharmaceutical sectors, our laboratory is equipped with cutting-edge technology and a team of highly trained professionals who ensure accurate, timely, and thorough testing results. These capabilities are part of Nikopharmad’s broader state-of-the-art laboratory network, dedicated to biocompatibility and toxicology testing for medical devices and pharmaceutical products.
Efficient and Timely Results
We understand the importance of time in product development. Our laboratory strives to deliver fast and reliable results, ensuring your products are tested and ready for market entry as efficiently as possible.
Confidentiality and Integrity
At Nikopharmad, we prioritize the confidentiality of your sensitive data and intellectual property. Our strict adherence to confidentiality agreements guarantees that your information remains protected throughout the testing process.
Global Compliance and Support
Whether you are preparing to enter the FDA, or other global markets, our laboratory ensures that your products comply with all necessary regulatory standards, facilitating a smooth path to market approval.
Choose Nikopharmad for Your Systemic toxicity testing Needs
By choosing Nikopharmad International Laboratory, you are selecting a trusted partner that not only meets but exceeds international testing standards. Our ISO 17025 certification, expert team, and commitment to excellence make us the ideal choice for ensuring the safety, compliance, and success of your medical devices and pharmaceutical products.
Reference: iso.org