What is MIC/MBC Testing?
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) testing are fundamental quantitative methods used to evaluate the antimicrobial activity of chemical agents, particularly antibiotics, against specific bacterial strains.
Minimum Inhibitory Concentration (MIC)
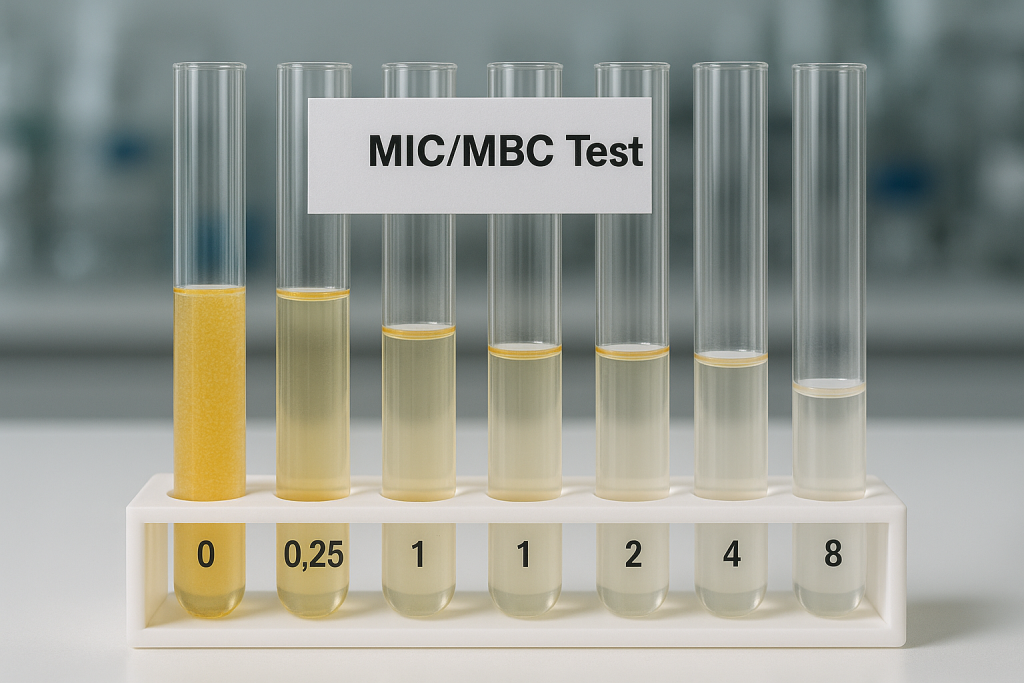
The MIC is defined as the lowest concentration of an antimicrobial agent that visibly inhibits the growth of a bacterial strain after a specified incubation period, typically 16–20 hours for most fast-growing organisms.
Standard Method (e.g., CLSI M07, M11):
MIC is typically determined using broth microdilution, agar dilution, or E-test methods. The broth microdilution method, considered the gold standard, involves:
Preparing a two-fold serial dilution series of the antimicrobial agent in a suitable broth medium (e.g., Mueller-Hinton broth).
Inoculating each well or tube with a standardized bacterial suspension (~5 × 10⁵ CFU/mL).
Incubating the plates/tubes at 35 ± 2°C for 16–20 hours under aerobic conditions.
Visually examining the wells for turbidity (growth). The MIC is the lowest concentration at which no visible growth is observed.
MIC values help guide appropriate antibiotic selection in clinical treatment and are also used in pharmacodynamic modeling and antibiotic resistance surveillance.
In many antimicrobial susceptibility workflows, MIC and MBC determinations are complemented by qualitative methods such as Kirby–Bauer disk diffusion testing and broader antibacterial testing screens to combine zone-of-inhibition data with precise quantitative concentration–response endpoints.
Minimum Bactericidal Concentration (MBC)
The MBC is defined as the lowest concentration of an antimicrobial agent that results in ≥99.9% (3-log) reduction in the initial bacterial inoculum, effectively killing the bacterial population rather than merely inhibiting it.
Standard Method (e.g., CLSI M26-A):
MBC testing is typically performed in conjunction with MIC determination:
After determining the MIC, aliquots from wells/tubes showing no visible growth (at or above the MIC) are subcultured onto drug-free agar plates.
The plates are incubated under the same conditions as the MIC test.
Colonies are counted after incubation. The MBC is the lowest antimicrobial concentration at which ≤0.1% of the original inoculum survives (i.e., ≥3-log₁₀ kill).
MBC testing differentiates between bactericidal and bacteriostatic activity. It is particularly useful in determining the appropriate antimicrobial therapy for immunocompromised patients or in serious infections where bactericidal activity is preferred (e.g., endocarditis, meningitis).
Interpretation and Clinical Relevance
Bacteriostatic vs. Bactericidal:
- If the MBC is ≤4 times the MIC, the antimicrobial is considered bactericidal.
- If the MBC is >4 times the MIC, it is typically regarded as bacteriostatic.
MIC and MBC data support dose optimization, resistance monitoring, and breakpoint determination by regulatory agencies and standards organizations.
MIC/MBC Testing Procedures
Objective
To quantitatively determine the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of an antimicrobial agent against a specific bacterial strain under standardized laboratory conditions.
Materials and Reagents
Bacterial strain: Pure culture, typically grown overnight.
Growth medium:
Mueller-Hinton broth (MHB) for aerobic and facultative anaerobes (CLSI-recommended).
Cation-adjusted MHB for specific bacteria requiring magnesium and calcium adjustments.
Antimicrobial agents: Pure standard powders diluted to working concentrations.
96-well sterile microtiter plates (for broth microdilution).
Agar plates: Mueller-Hinton agar (MHA) for MBC plating.
Spectrophotometer (optional) for OD600 readings (turbidity).
Incubator set to 35 ± 2°C.
Inoculum Preparation
From an overnight culture, a bacterial suspension is prepared in sterile saline or broth.
Adjust turbidity to match 0.5 McFarland standard (≈1.5 × 10⁸ CFU/mL).
Further dilute the suspension in broth medium to reach a final inoculum concentration of ~5 × 10⁵ CFU/mL in each well (for MIC).
MIC Procedure (Broth Microdilution Method)
Antibiotic dilution
Prepare a serial two-fold dilution series of the antimicrobial agent in the broth medium (e.g., 64, 32, 16, … 0.125 µg/mL).
Dispense 100 µL of each dilution into wells of a 96-well microtiter plate.
Inoculation
Add 100 µL of standardized bacterial inoculum to each well, resulting in 200 µL final volume per well.
Controls
Positive control: Medium + inoculum (no antibiotic).
Negative control: Medium + antibiotic (no inoculum) to check sterility.
Media control: Media only (background correction).
Incubation
Incubate microtiter plates at 35 ± 2°C for 16–20 hours (for fast-growing aerobic bacteria).
Anaerobic or slow-growing organisms may require up to 48 hours under appropriate conditions.
Interpretation
Visually inspect each well for turbidity (growth).
MIC is the lowest concentration where no visible growth (clear well) is observed.
MBC Procedure (Subculture Method)
Performed immediately after MIC determination:
From wells with no visible growth (at or above MIC), take 10 µL aliquots and plate onto drug-free Mueller-Hinton agar.
Spread inoculum evenly and incubate at 35 ± 2°C for 24–48 hours.
After incubation:
Count colony-forming units (CFU).
The MBC is the lowest antibiotic concentration at which ≥99.9% of the original inoculum is killed, meaning ≤0.1% survival (i.e., 3-log₁₀ CFU/mL reduction compared to original inoculum).
Original inoculum count must also be plated and recorded to determine log reduction.
Data Analysis and Interpretation
Bacteriostatic vs. Bactericidal:
If MBC/MIC ≤ 4, the agent is considered bactericidal.
If MBC/MIC > 4, the agent is considered bacteriostatic.
Example:
MIC = 1 µg/mL, MBC = 2 µg/mL → bactericidal (MBC/MIC = 2).
MIC = 1 µg/mL, MBC = 16 µg/mL → bacteriostatic (MBC/MIC = 16).
Breakpoints
MIC results can be compared with CLSI or EUCAST interpretive breakpoints to categorize susceptibility: Susceptible (S), Intermediate (I), or Resistant (R).
Quality Control
Quality Control Strains:
Commonly used: E. coli ATCC 25922, S. aureus ATCC 29213, P. aeruginosa ATCC 27853.
Ensure results fall within published QC ranges.
Ensure reagents, incubation conditions, and media meet validated specifications.
Applications
Clinical antibiotic susceptibility profiling.
Pharmaceutical antimicrobial efficacy screening.
Determining dose-response for novel antimicrobial candidates.
Validating biocidal product claims.
Research on resistance mechanisms.
antifungal agents(For yeast and mould isolates, MIC endpoints can be extended to antifungal agents, and are often run in parallel with dedicated antifungal activity testing to fully characterise fungistatic and fungicidal performance).
Summary
| Parameter | MIC | MBC |
|---|---|---|
| Definition | Lowest concentration to inhibit growth | Lowest concentration to kill ≥99.9% bacteria |
| Method | Broth microdilution (visual or spectrophotometric) | Subculture from MIC tubes to agar |
| Readout | Absence of turbidity | No colony growth or ≥3-log reduction |
| Time | 16–20 h (aerobes) | 24–48 h post-subculture |
| Output | µg/mL | µg/mL |
| Interpretation | Antimicrobial potency | Bacteriostatic vs. bactericidal classification |
MBC and MIC testing, when performed according to established standards like CLSI M07 and M26, provide essential, reproducible data for understanding antimicrobial activity, supporting regulatory submissions, clinical decisions, and R&D for new therapeutics.
Why Choose Nikopharmad for Your MIC/MBC Testing Needs?
ILAC Accreditation & ISO/IEC 17025 Certification
Nikopharmad Laboratory is fully accredited under the ILAC Mutual Recognition Arrangement and certified to ISO/IEC 17025 standards, ensuring that our MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) testing services meet globally accepted standards of analytical accuracy, scientific reproducibility, and technical rigor.
Comprehensive Antimicrobial Susceptibility Testing Services
Nikopharmad offers a full suite of validated MIC and MBC testing services based on gold-standard methodologies established by CLSI (e.g., M07-A11, M26-A) and EUCAST guidelines. Our protocols are designed to determine the inhibitory and bactericidal concentrations of antimicrobial agents against a wide variety of bacterial pathogens and product-associated isolates.
Expert Scientific Staff & Advanced Laboratory Infrastructure
Our MIC/MBC testing services are led by a team of highly qualified microbiologists, biochemists, and pharmaceutical scientists with extensive expertise in antimicrobial susceptibility testing, resistance profiling, and method development.
Accelerated Timelines with Validated Methodologies
Nikopharmad understands the importance of timely antimicrobial evaluation in product development and clinical pipeline decisions. Our validated MIC/MBC methods are optimized to provide rapid turnaround, supporting early-phase screening, batch testing, and formulation refinement.
Competitive & Transparent Pricing
We are committed to providing cost-effective MIC/MBC testing services with transparent pricing. Whether you’re an early-stage biotech or an established pharmaceutical manufacturer, we offer flexible service packages, volume-based discounts, and long-term engagement models designed to accommodate both R&D and commercial-scale requirements—without compromising quality.
Data Security & Intellectual Property Protection
Your intellectual property is our highest priority. Nikopharmad enforces robust data security protocols, electronic records management systems, and binding non-disclosure agreements (NDAs) to protect sensitive formulations and test results. Our audit-ready documentation complies with FDA 21 CFR Part 11, GxP standards, and international data integrity expectations.
Regulatory Compliance & Global Submission Support
Our MIC/MBC reports are fully aligned with regulatory expectations from CLSI, EUCAST, ISO 20776, and ICH guidelines. Each report includes detailed method descriptions, validation summaries, quality control results, and interpretive criteria (e.g., bacteriostatic vs. bactericidal classification), making them suitable for submission to global health authorities.
To request testing or a complimentary consultation contact Nikopharmad
Partner with Nikopharmad for Accurate, Compliant, and Cost-Effective MIC/MBC Testing
By choosing Nikopharmad for your MIC/MBC testing needs, you gain a trusted laboratory partner with proven scientific expertise, rapid delivery timelines, regulatory-grade quality systems, and competitive pricing. Our precise susceptibility profiling ensures your antimicrobial products and ingredients are evaluated with the highest level of scientific rigor—ready for confident decision-making and regulatory advancement.