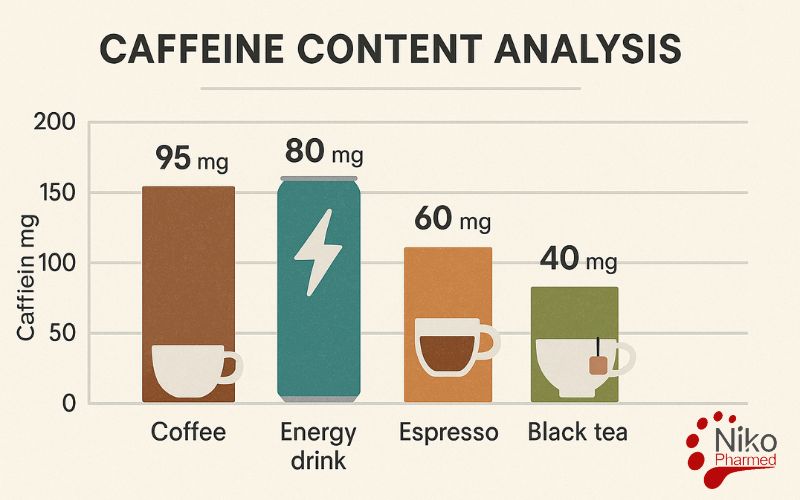
What is Caffeine Content Analysis?
Caffeine Content Analysis, is the process of determining the precise concentration of caffeine in a product such as beverages, dietary supplements, or pharmaceuticals using methodologies that comply with globally recognized frameworks.
Results are expressed in standardized units (e.g., mg/100 mL or mg/g) with stated measurement uncertainty, ensuring that data are reliable, comparable across laboratories, and compliant with regulatory and labeling requirements for safety, quality, and consumer protection.
Caffeine Content Analysis Procedure Test Methods
Sampling and Preparation
According to ISO guidelines (e.g., ISO 1446 for coffee, ISO 3103 for tea), representative sampling is essential to avoid bias. Samples must be stored in moisture-proof, light-resistant containers at controlled temperatures to prevent degradation or caffeine loss. Grinding or homogenizing is performed under conditions that do not alter caffeine content.
Analytical Methods
International standards recognize several validated methods for caffeine determination, each chosen based on matrix complexity, required sensitivity, and regulatory needs:
- High-Performance Liquid Chromatography (HPLC)
Standard References: ISO 20481 (tea and coffee), USP <1225>, AOAC 995.03.
Principle: Caffeine is extracted using an aqueous or aqueous-organic solvent, separated via a chromatographic column, and detected using ultraviolet (UV) absorbance (typically at 272–280 nm).
Advantages: High sensitivity, selectivity, and applicability to complex matrices.
- UV–Visible Spectrophotometry
Standard References: AOAC 979.08 for soft drinks.
Principle: Caffeine is extracted and its absorbance measured at a characteristic wavelength (usually 272–275 nm).
Advantages: Rapid and cost-effective for simple matrices with minimal interference.
- Liquid Chromatography–Mass Spectrometry (LC–MS)
Standard References: Applied in high-precision research and regulatory labs; method harmonized with ISO/IEC 17025 requirements.
Principle: Combines liquid chromatography separation with mass spectrometric detection for enhanced sensitivity and selectivity, allowing analysis at trace levels.
Advantages: Excellent specificity and capability to detect caffeine in highly complex matrices or trace concentrations.
- Gravimetric/Titrimetric Methods (Historical/Legacy)
Standard References: Limited in current ISO usage; more common in older pharmacopoeial methods.
Principle: Caffeine is isolated and quantified based on its weight or chemical reaction with a titrant.
Advantages: Simple equipment requirements but low sensitivity and specificity.
Calibration and Quality Control
All methods require calibration against certified reference materials (CRMs) to ensure traceability to SI units. Quality control includes blank runs, spiked recovery tests, and participation in inter-laboratory proficiency schemes to confirm accuracy and reproducibility.
Data Reporting
Results must be expressed in mg of caffeine per defined unit (e.g., mg/100 mL for liquids, mg/g for solids), with associated measurement uncertainty stated in compliance with ISO/IEC Guide 98-3 (GUM). Method references, validation data, and uncertainty estimates must be included in the final analytical report.
Method Comparison Table
| Method | Sensitivity | Selectivity | Speed | Cost | Matrix Complexity Handling | Typical Application |
|---|---|---|---|---|---|---|
| HPLC (UV detection) | High | High | Moderate | Moderate | High | Beverages, foods, pharmaceuticals |
| UV–Vis Spectrophotometry | Moderate | Low–Moderate | Fast | Low | Low–Moderate | Soft drinks, simple extracts |
| LC–MS | Very High | Very High | Moderate–Slow | High | Very High | Trace detection, complex matrices, forensic analysis |
| Gravimetric/Titrimetric | Low | Low | Slow | Low | Low | Historical methods, basic labs without advanced equipment |
Nikopharmed Caffeine Content Analysis laboratory
Laboratory Accreditation and Global Recognition
At Nikopharmad, our Caffeine Content Analysis services are backed by ISO/IEC 17025 certification and ILAC accreditation, ensuring strict adherence to globally recognized analytical quality and technical competence standards. These credentials guarantee that our caffeine determinations meet the most rigorous international regulatory requirements and are recognized by AOAC International, ISO standards, USP, Ph. Eur., and leading food, beverage, pharmaceutical, and nutritional supplement regulatory authorities worldwide.
Technical Excellence and Validated Infrastructure
Our state-of-the-art analytical facility is equipped with high-performance liquid chromatography (HPLC) systems with UV and diode-array detection, LC–MS/MS platforms for ultra-trace analysis, precision analytical balances, automated sample preparation systems, and validated extraction apparatus. We are capable of analyzing caffeine content in a wide range of matrices—from coffee, tea, and energy drinks to dietary supplements, pharmaceuticals, and functional foods.
Regulatory-Ready Reporting and Timely Results
In industries where compliance timelines are critical, Nikopharmad delivers rapid, accurate, and defensible caffeine content results. All analytical reports include full method validation records, traceable calibration data, and statistical analysis aligned with FDA, Codex Alimentarius, European Commission, and other international regulatory submission requirements.
Our optimized workflow ensures fast turnaround times without compromising analytical precision or compliance, supporting quality assurance programs, product certification, import/export inspections, and label claim verification.
Confidentiality and Data Integrity
We uphold the highest levels of client confidentiality and data security. All caffeine content testing activities are carried out under strict non-disclosure agreements and supported by 21 CFR Part 11-compliant electronic data systems. Every analysis is documented with a complete audit trail, ensuring traceability, reliability, and integrity of results. Proprietary formulations, research data, and regulatory submissions are handled with uncompromising ethical and professional standards.
Competitive Pricing with Uncompromised Quality
We offer cost-effective caffeine analysis solutions tailored to the needs of small enterprises, global corporations, and regulatory agencies. Our transparent pricing structure allows clients to meet compliance requirements efficiently while upholding international analytical standards reducing approval timelines without compromising the quality or reliability of results.
To request testing or a complimentary consultation contact Nikopharmad
Partner with Nikopharmad for Expert Caffeine Content Analysis
By partnering with Nikopharmad, you gain access to a globally accredited laboratory dedicated to scientific excellence, regulatory compliance, and data confidentiality. Whether ensuring product consistency, verifying label claims, meeting market-entry regulations, or satisfying strict industry standards, our caffeine content analysis expertise guarantees accurate, reliable, and internationally recognized results, helping you achieve compliance faster and with complete confidence.