What is Sucrose Analysis?
Sucrose analysis refers to the quantitative determination of sucrose content in a sample, typically in food, pharmaceutical, or biochemical matrices. The objective is to accurately measure sucrose concentration using validated analytical methods that comply with recognized standards to ensure reliability, reproducibility, and traceability.
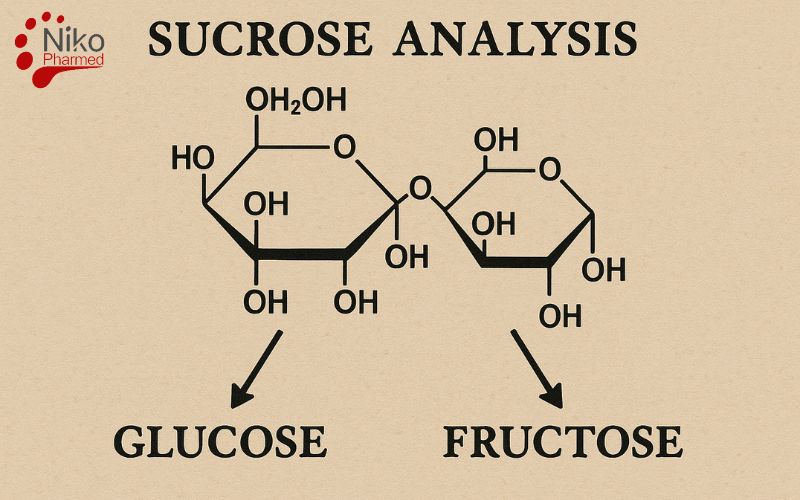
As a disaccharide composed of glucose and fructose (C₁₂H₂₂O₁₁), sucrose plays a significant role in food science, agriculture, pharmaceuticals, and biochemical research. Accurate sucrose analysis is essential for ensuring compliance with quality control requirements, nutritional labeling regulations, and trade specifications, as well as for detecting adulteration or changes in product integrity.
In food and beverage products, sucrose content data are typically evaluated together with Total Sugar Content Analysis and Carbohydrate Analysis to verify nutrition label claims, support product reformulation and ensure compliance with dietary regulations.
Following standardized global protocols ensures that analytical results are precise, reproducible, and comparable across laboratories and jurisdictions, thereby safeguarding consumer trust and supporting international commerce.
Sucrose Analysis Procedure Test Methods
Sucrose analysis refers to the quantitative determination of sucrose content in a sample, typically in food, pharmaceutical, or biochemical matrices. The objective is to accurately measure sucrose concentration using validated analytical methods that comply with recognized standards to ensure reliability, reproducibility, and traceability.
International standards such as ISO and USP define validated test methods to control for:
Accuracy (closeness to the true value)
Precision (repeatability and reproducibility)
Specificity (ability to measure sucrose without interference from other sugars)
Linearity and range
Detection limits
Standard reference materials
Relevant Standards
ISO Standards
Several ISO standards address sucrose determination depending on the sample type:
- ISO 10565 : Sugar – Determination of sugar content by polarimetric methods.
- ISO 11292 : Sugar – Determination by HPLC with refractive index detection.
- ISO 17323 : Cane raw sugar – Polarimetric sucrose content.
USP Standards
- USP–NF Monographs specify sucrose assay procedures for pharmaceutical-grade sucrose.
- USP <541> (Sucrose Assay) details polarimetric methods.
- USP General Chapter <621> Chromatography governs HPLC method validation parameters.
Common Internationally Recognized Test Methods
Polarimetry (Optical Rotation Method)
Sucrose is optically active, rotating plane-polarized light to the right. Polarimetry measures the angle of rotation, which is proportional to sucrose concentration in solution, under specified temperature, wavelength (sodium D-line, 589.3 nm), and path length conditions.
Procedure (ISO / USP guidelines)
Sample preparation : Dissolve an accurately weighed sample in distilled water or specified solvent.
Clarification :Remove color and turbidity (often with lead acetate or modern non-toxic clarifying agents).
Polarimetric measurement : Using a calibrated polarimeter, record rotation.
Calculation
Sucrose (%)= 100×(observed rotation) / specific rotation of sucrose at reference conditions
(Specific rotation for sucrose at 20°C: +66.47° in water)
Advantages:
Rapid, simple, non-destructive.
Limitations:
Cannot distinguish sucrose from other optically active sugars unless combined with inversion (acid hydrolysis) and difference measurement.
High-Performance Liquid Chromatography (HPLC)
HPLC separates sucrose from other sugars using a carbohydrate column (often amine or calcium-based) and quantifies it by refractive index (RI) or evaporative light scattering (ELSD) detection.
Procedure
Sample preparation – Filtration and dilution in water or aqueous acetonitrile.
Chromatographic separation – Mobile phase: acetonitrile/water (75:25 to 85:15), isocratic run.
Detection and calibration – Compare retention time and peak area against certified sucrose standards.
Quantitation – External standard calibration curve.
Advantages:
High specificity, can separate sucrose from glucose, fructose, and other carbohydrates.
Limitations:
Requires expensive equipment and longer run time.
Enzymatic Method
Sucrose is hydrolyzed by invertase to glucose and fructose. Glucose is quantified using glucose oxidase/peroxidase coupled colorimetric assays, and sucrose is calculated by difference(In many laboratories, these colorimetric reactions are monitored using UV-Vis spectroscopy, enabling precise quantification of sucrose via its enzymatically generated chromophores.)
Advantages:
Highly specific.
Limitations:
Enzyme stability, reagent cost.
Validation and Quality Control
Both ISO and USP require:
Calibration with traceable reference standards (e.g., NIST SRM 17c sucrose)
Repeatability testing (intra-day precision)
Reproducibility testing (inter-day, inter-laboratory precision)
Recovery studies to confirm accuracy
Limit of detection (LOD) and limit of quantitation (LOQ) determination
Measurement uncertainty estimation
Scientific Considerations
Temperature control is critical for polarimetry (specific rotation changes with temperature).
pH stability must be maintained to prevent sucrose hydrolysis during analysis(Where pH adjustment or verification is required, sucrose assays are often supported by dedicated pH Testing to ensure the sample remains within the validated range throughout preparation and measurement).
Sample matrix effects (e.g., colorants, proteins) must be removed or compensated for.
Standard addition method is often used in complex matrices to counter matrix interference.
Nikopharmed Sucrose Content Analysis Laboratory
Laboratory Accreditation and Global Recognition
At Nikopharmed, our Sucrose Content Analysis services are backed by ISO/IEC 17025 certification and ILAC accreditation, ensuring strict adherence to globally recognized analytical quality and technical competence standards.
These credentials guarantee that our sucrose determinations meet the most rigorous international regulatory requirements and are recognized by AOAC International, ISO standards, USP, Ph. Eur., and leading food, beverage, pharmaceutical, and nutritional supplement regulatory authorities worldwide.
Technical Excellence and Validated Infrastructure
Our state-of-the-art analytical facility is equipped with polarimetric systems for official trade and quality control assays, high-performance liquid chromatography (HPLC) with refractive index and evaporative light scattering detection for high specificity, and enzymatic analysis platforms for selective determinations in complex matrices.
We maintain precision analytical balances, automated sample preparation systems, and validated clarification and extraction apparatus.
We are capable of analyzing sucrose content in a wide range of matrices—from refined sugar, syrups, and confectionery to pharmaceutical formulations, functional foods, beverages, and nutritional supplements.
Regulatory-Ready Reporting and Timely Results
In industries where compliance timelines are critical, Nikopharmed delivers rapid, accurate, and defensible sucrose content results. All analytical reports include:
Full method validation records
Traceable calibration data using certified reference standards (e.g., NIST SRM sucrose)
Statistical analysis aligned with FDA, Codex Alimentarius, European Commission, and other international regulatory submission requirements.
Our optimized workflow ensures fast turnaround times without compromising analytical precision or compliance, supporting quality assurance programs, product certification, import/export inspections, and label claim verification.
Confidentiality and Data Integrity
We uphold the highest levels of client confidentiality and data security. All sucrose content testing activities are carried out under strict non-disclosure agreements and supported by 21 CFR Part 11-compliant electronic data systems. Every analysis is documented with a complete audit trail, ensuring traceability, reliability, and integrity of results. Proprietary formulations, research data, and regulatory submissions are handled with uncompromising ethical and professional standards.
Competitive Pricing with Uncompromised Quality
We offer cost-effective sucrose analysis solutions tailored to the needs of small enterprises, global corporations, and regulatory agencies. Our transparent pricing structure enables clients to meet compliance requirements efficiently while upholding international analytical standards, reducing approval timelines without compromising the quality or reliability of results.
To request testing or a complimentary consultation contact Nikopharmad
Partner with Nikopharmed for Expert Sucrose Content Analysis
By partnering with Nikopharmed, you gain access to a globally accredited laboratory dedicated to scientific excellence, regulatory compliance, and data confidentiality. Whether ensuring product consistency, verifying label claims, meeting market-entry regulations, or satisfying strict industry standards, our sucrose content analysis expertise guarantees accurate, reliable, and internationally recognized results—helping you achieve compliance faster and with complete confidence.