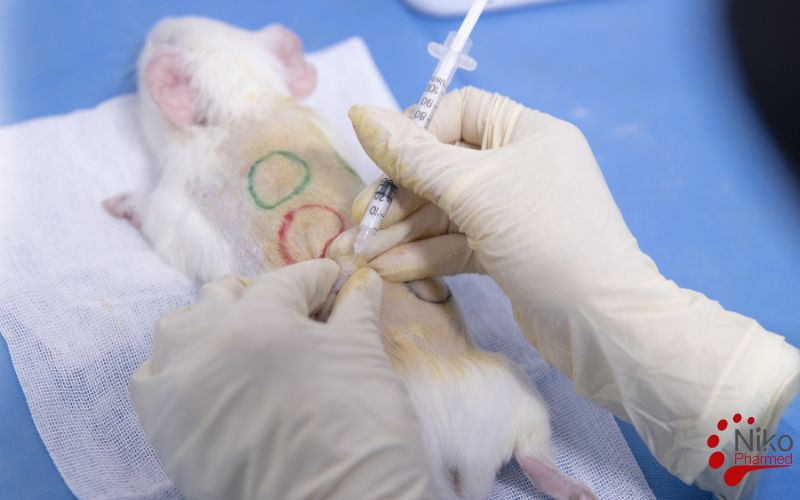
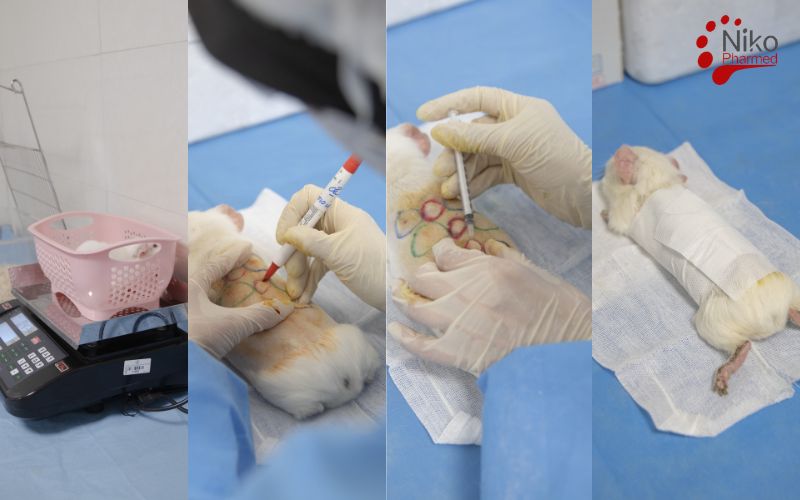
What is Sensitization Testing?
The sensitization testing as outlined in ISO 10993-10 is a biological evaluation used to assess the potential of a material or medical device to cause allergic reactions or hypersensitivity in living organisms. The test primarily aims to determine whether a material induces an immune response after repeated exposure. This is typically tested using animal models, most commonly guinea pigs, which are exposed to the material in a controlled manner. The procedure involves an initial sensitization phase, followed by a challenge phase, where the material is re-applied to the animal’s skin.
The test observes any signs of allergic reactions, such as redness, swelling, or other immune system responses. The results help determine if the material is safe for use in medical devices that will come into contact with human skin or mucosa, ensuring that it does not provoke an adverse immune reaction. Within the ISO 10993 biological evaluation framework, sensitization studies are interpreted together with irritation testing for skin- and mucosa-contacting medical devices.
Sensitization(Hypersensitivity) Testing Procedure
Selection of Test Material
The material or medical device to be tested is chosen. It should be in its final form, as it will be used in clinical settings.
The material should represent the intended product, including any processing, coatings, or surface treatments.
Animal Selection and Preparation
The test is typically conducted using guinea pigs, which are selected based on their immune system’s response to allergens.
The animals should be healthy, acclimatized, and of similar age and weight to ensure consistency in results.
Hair is typically shaved from the test site (e.g., the back) to ensure direct contact between the material and the skin.
Induction Phase (Sensitization Phase)
In the induction phase, the material is applied to the test animals for the first time. This is done to provoke an immune response, typically using a concentration of the material in a suitable vehicle (e.g., a solution or ointment).
The material is usually applied to a small area of the skin (e.g., the shaved back of the guinea pig), and the animals are monitored for any signs of irritation or reaction.
The exposure period is typically 1 to 3 weeks depending on the testing protocol. During this time, the material is applied two or more times in a specific regimen, usually with an interval of several days between applications.
Challenge Phase
After the induction phase, a challenge phase is performed, typically 2 weeks after the last application in the sensitization phase.
In this phase, the material is re-applied to the skin of the animals, but this time it is applied to a new area that was not previously exposed during the induction phase.
The goal of the challenge phase is to observe if the immune system reacts to the material after it has been sensitized.
Observation for Reactions
Following the challenge phase, the animals are observed for signs of allergic reactions such as erythema (redness), edema (swelling), or any other symptoms of inflammation.
The response is carefully monitored and documented for several days after the challenge exposure.
If signs of allergic reactions or hypersensitivity appear (e.g., skin swelling or redness), the material is considered to have sensitization potential.
Scoring and Evaluation
The observed reactions are scored according to severity, typically using a graded scale (e.g., 0 to 4), with higher scores indicating more severe reactions.
A positive result is typically indicated if there is a clear allergic response (e.g., swelling, redness) that is significantly greater than the initial baseline condition.
Negative results are noted if no reaction occurs or the response is minimal.
Interpretation of Results
The material is classified as either a sensitizer or non-sensitizer based on the outcome of the test.
-
-
- Sensitizer: If the material induces an allergic response in a significant number of test animals.
- Non-sensitizer: If no allergic reaction or only mild reactions are observed, and the material does not induce hypersensitivity.
-
Reporting and Documentation
The findings from the sensitization test, including the type and severity of reactions, should be documented and reported as per the standard.
Any recommendations for material modification or further testing should be included if significant sensitization is observed.
To request testing or a complimentary consultation contact Nikopharmad
Importance of Sensitization(Hypersensitivity) Testing
The importance of sensitization testing is grounded in several technical and regulatory imperatives:
Biological Safety Assurance
Sensitization responses are immune-mediated and can manifest even at trace exposure levels. In vivo assays, including the Guinea Pig Maximization Test and Buehler Test, remain gold standards for detecting such effects and are designed to reflect real-world biological interactions.
Regulatory Compliance
This testing is mandated by ISO 10993-10 and is required by authorities such as the FDA and EU regulators. Devices that fail to meet sensitization safety criteria cannot proceed to market.
Early Risk Mitigation
Identifying sensitization potential early in the product development phase prevents costly recalls and protects brand reputation. My experience confirms that integrating sensitization testing into the early design verification phase significantly streamlines regulatory review and reduces long-term liabilities.
Sensitization or Hypersensitivity Testing Lab
At International & Accredited Lab Nikopharmad Laboratory Network, we are committed to providing accurate, reliable, and high-quality biocompatibility testing services for medical devices and pharmaceutical products. Holding the esteemed ISO 17025 certification and ILAC accreditation, we adhere to internationally recognized standards, ensuring that our testing processes meet the highest quality and regulatory requirements.
Why Choose Nikopharmad Laboratory?
ILAC accreditation
Nikopharmad Laboratory is proudly certified with ISO 17025 and holds ILAC accreditation. These prestigious certifications ensure that our laboratory operates with the highest standards of quality and competence, providing reliable and internationally recognized testing services for medical devices and pharmaceutical products. Our commitment to maintaining these certifications demonstrates our dedication to excellence and compliance with global regulatory requirements.
Comprehensive Testing Capabilities
We offer a broad range of biocompatibility tests , each designed to assess the safety and performance of your products in compliance with international regulatory frameworks.
Expertise and Experience
With extensive experience in the medical and pharmaceutical sectors, our laboratory is equipped with cutting-edge technology and a team of highly trained professionals who ensure accurate, timely, and thorough testing results.
Efficient and Timely Results
We understand the importance of time in product development. Our laboratory strives to deliver fast and reliable results, ensuring your products are tested and ready for market entry as efficiently as possible.
Confidentiality and Integrity
At Nikopharmad, we prioritize the confidentiality of your sensitive data and intellectual property. Our strict adherence to confidentiality agreements guarantees that your information remains protected throughout the testing process.
Global Compliance and Support
Whether you are preparing to enter the FDA, or other global markets, our laboratory ensures that your products comply with all necessary regulatory standards, facilitating a smooth path to market approval.
Reference:iso.org