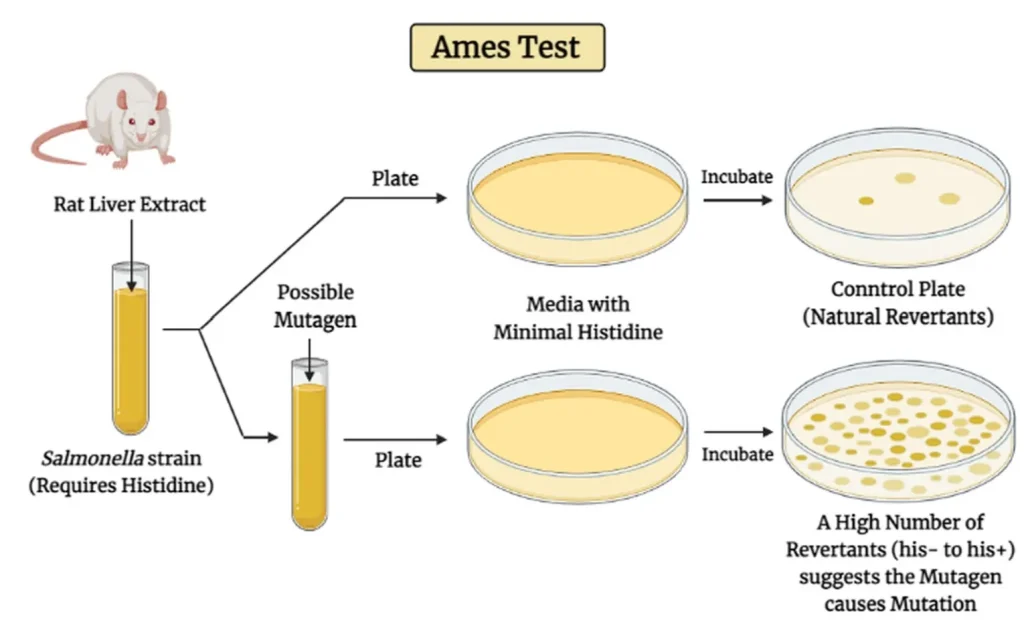
What Is Ames Test?
The Ames Test is a standardized in vitro assay designed to assess the mutagenic potential of chemical substances that may leach from medical devices. This bacterial reverse mutation assay employs specific strains of Salmonella typhimurium (and sometimes Escherichia coli) that carry mutations rendering them unable to synthesize an essential amino acid (typically histidine or tryptophan). The Ames Test is a crucial method for Genotoxicity Testing, assessing whether medical device materials may cause genetic damage when in contact with mammalian cells
ISO 10993-3 requires the use of both with and without metabolic activation systems (e.g., S9 mix) to simulate in vivo conditions and detect both direct-acting and bioactivated mutagens. The test must be conducted using appropriate positive and negative controls, multiple bacterial strains with differing mutation spectra, and in accordance with Good Laboratory Practice (GLP). It serves as an initial screening tool in the overall biological risk assessment strategy for evaluating the safety of medical devices.
The Ames Test procedure
The Ames Test evaluates the mutagenic potential of a test substance by observing its ability to induce reverse mutations in histidine-dependent strains of Salmonella typhimurium (such as TA98, TA100, TA1535, TA1537) and optionally Escherichia coli WP2 strains (e.g., WP2 uvrA).
Preparation of Test System
Select at least four to five bacterial strains with different mutation spectra. These strains are chosen for their sensitivity to different types of mutagens (frameshift and base-pair substitutions). All strains must be characterized and quality-controlled for their genetic markers.
Metabolic Activation System (S9 mix)
Since some compounds become mutagenic only after metabolic transformation, the test must be conducted both with and without a metabolic activation system. This is typically a rat liver microsomal enzyme fraction (S9) induced with Aroclor 1254 or a similar inducer.
Test Article Preparation
For medical devices, extracts of the device material are prepared using polar (e.g., saline or cell culture media) and non-polar (e.g., corn oil or DMSO) solvents under conditions described in ISO 10993-12. The choice of extraction vehicle, time, and temperature should reflect the intended clinical use of the device and its physicochemical characteristics.
Treatment Conditions
A plate incorporation method or pre-incubation method is used:
Plate Incorporation: Bacteria, test article (or extract), S9 mix (if used), and top agar are mixed and poured onto minimal agar plates.
Pre-Incubation: Bacteria, test article, and S9 mix are incubated for ~20–30 minutes at 37°C before plating.
Controls
Negative controls: solvent or extract vehicle.
Positive controls: known mutagens appropriate for each strain (e.g., sodium azide for TA100 without S9, 2-aminoanthracene for TA98 with S9).
Solvent controls are also used for the extract vehicle to rule out vehicle-induced effects.
Dose Levels and Replication:
At least five dose levels of the test article (or extract) are tested, with triplicate plates per dose, to establish a dose-response relationship and identify cytotoxicity. The highest dose should produce some cytotoxicity or precipitation but not complete bacterial killing.
Incubation and Scoring
Plates are incubated at 37°C for 48–72 hours, after which revertant colonies are manually or automatically counted. A positive result is indicated by a dose-dependent increase in revertant colonies that is at least twice the background level and statistically significant.
Data Interpretation
Results must be interpreted using both biological relevance (dose-response and reproducibility) and statistical significance. A positive finding in any strain, particularly if confirmed in repeat experiments, indicates mutagenic potential and warrants further risk assessment or additional in vitro or in vivo genotoxicity studies.
Reporting Requirements
The final report must include full details of:
- Bacterial strains and their genotypic characteristics.
- Test article preparation and extraction methods.
- Concentrations and solvents used.
- Control data.
- Number of revertant per plate and standard deviations.
- Statistical analysis and justification of result interpretation.
The Ames test under ISO 10993-3 is an essential early-stage assay for identifying genotoxic risks associated with medical device materials. It is not standalone and must be interpreted within a weight-of-evidence approach alongside other genotoxicity assays (e.g., chromosomal aberration or micronucleus tests) as part of a comprehensive biological risk assessment strategy. Also The Ames Test is widely used for Genotoxicity Testing, helping to identify potential genotoxic substances in materials before clinical use
Ames Test at Nikopharmad Laboratory
At Nikopharmad Laboratory Network, we deliver precise, reliable, and globally compliant mutagenicity testing using the Ames Test (Bacterial Reverse Mutation Test)—a cornerstone assay for evaluating the genotoxic potential of pharmaceuticals, medical devices, chemicals, and raw materials. Accredited under ISO/IEC 17025 and recognized by ILAC, our facility operates to the highest international standards, ensuring the integrity, accuracy, and regulatory acceptance of all test results.
To request testing or a complimentary consultation contact Nikopharmad
Why Choose Nikopharmad for Your Ames Test Needs?
ILAC Accreditation & ISO 17025 Certification
Our laboratory is accredited by ILAC and operates under ISO 17025 guidelines, validating both our technical competence and quality management system. This ensures your Ames test data is recognized by global regulatory agencies such as the FDA, EMA, TÜV, and MHLW, significantly streamlining your approval process.
Comprehensive Mutagenicity Testing Capabilities
We offer the Ames test under OECD Test Guideline 471, covering multiple Salmonella typhimurium strains (e.g., TA98, TA100, TA1535, TA1537, TA102), with and without S9 metabolic activation, to detect both direct and metabolically-activated mutagens.
Scientific Expertise and State-of-the-Art Facilities
Our team of toxicologists, microbiologists, and regulatory scientists combine decades of experience with advanced lab infrastructure—including automated colony counters, certified S9 mix preparations, and validated 96-well MPF formats—for both qualitative and quantitative analysis.
Rapid Turnaround Time with Validated Protocols
Time-to-market is critical. Our streamlined workflows, risk-based batching, and optimized pre-incubation protocols ensure fast and reproducible test outcomes—without compromising on scientific rigor or regulatory acceptance.
Data Confidentiality and IP Integrity
We strictly enforce confidentiality policies, non-disclosure agreements (NDAs), and secure data handling protocols to ensure your proprietary formulations and results are fully protected throughout the testing lifecycle.
Regulatory Compliance and Global Market Access
Our test reports are formatted for immediate submission to regulatory bodies worldwide, supporting dossiers under ISO 10993-3, ICH S2(R1), and REACH. Whether you’re targeting FDA, CE, or PMDA approval, our Ames testing platform aligns with the most stringent international guidelines.
Partner with Nikopharmad for Ames Test
By choosing Nikopharmad Laboratory for Ames Test services, you are selecting a globally trusted, ISO-accredited partner with deep expertise in genetic toxicology. Our commitment to scientific excellence, rapid delivery, and global compliance ensures your innovations reach the market safely and successfully.
Differences Between Ames Test and HPRT
The Ames test and the HPRT (Hypoxanthine-Guanine Phosphoribosyltransferase) gene mutation assay are both in vitro genotoxicity tests used to detect gene mutations, but they differ significantly in terms of test system, target gene, sensitivity to mutagen classes, and metabolic competence. A thorough understanding of their methodological and mechanistic differences is critical for accurate interpretation in regulatory and toxicological contexts.
Test System and Genetic Endpoint
- Ames Test:
Utilizes prokaryotic test organisms—specifically histidine-dependent strains of Salmonella typhimurium (e.g., TA98, TA100) and sometimes Escherichia coli (e.g., WP2 uvrA).
Detects reverse mutations (back mutations) in specific loci, allowing the bacteria to grow in histidine-deficient media. - HPRT Assay:
Performed in mammalian cells, typically Chinese Hamster Ovary (CHO) or V79 cells, or human lymphocytes.
Measures forward mutations at the HPRT locus, where mutations in the purine salvage pathway confer resistance to 6-thioguanine (6-TG), a cytotoxic purine analog.
Mutation Spectrum Sensitivity
Ames Test is highly sensitive to base-pair substitutions and frameshift mutations. For example:
TA98: detects frameshifts.
TA100: detects base substitutions.
It is less effective for detecting large deletions or aneuploidy.
HPRT Assay detects a broader range of genetic events, including base substitutions, small deletions, and occasionally larger chromosomal alterations affecting gene function. It reflects mutation processes more representative of mammalian systems.
Metabolic Activation
- Both tests often employ an exogenous metabolic activation system (S9 mix) to simulate in vivo metabolism.
- However, HPRT, being a mammalian cell system, may also possess endogenous metabolic capability—although less efficient than hepatocyte-derived enzymes. This intrinsic capacity is particularly relevant for substances requiring bioactivation not adequately mimicked by bacterial systems.
Relevance to Human Risk
- The Ames Test, while highly predictive for bacterial mutagens, may produce false positives or false negatives when extrapolated to human biology due to the differences in DNA repair pathways, membrane permeability, and chromatin structure.
- HPRT, using mammalian cells with eukaryotic DNA repair and cell cycle control, provides better physiological relevance to human genetic toxicology, albeit with lower throughput and longer assay duration (7–14 days compared to 2–3 days for Ames).
Regulatory Use and Complementarity
According to OECD guidelines and ISO 10993-3, both tests are recommended for a tiered genotoxicity assessment:
- Ames is typically used as a first-line screen due to its speed, cost-effectiveness, and sensitivity.
- HPRT serves as a follow-up or confirmatory assay, particularly for substances that are negative in the Ames test but structurally suspect or likely to act via mammalian-specific pathways.
Scientific Data Example
A large retrospective study by Kirkland et al. (2005) analyzing 730 chemicals reported that:
- The Ames test detected 58% of rodent carcinogens.
- The HPRT test alone detected 72% of the same group.
Combining the two assays increased the predictive accuracy to over 80% for in vivo genotoxicity.
This illustrates the complementary value of these assays and supports a battery approach to genotoxicity testing.
In conclusion, the Ames Test is a crucial component in assessing the genotoxicity of materials used in medical devices. It helps identify potential risks and ensures that products meet ISO 10993 standards for safety and compliance. To ensure the reliability and accuracy of your testing, Contact Nikopharmad Laboratory for expert Ames testing services.
Reference:iso.org