What is Comet Assay?
The Comet Assay, also known as single-cell gel electrophoresis, is a sensitive and versatile method used to detect DNA strand breaks at the level of individual cells, and is recognized under ISO 10993-3 for the assessment of genotoxicity in medical devices.
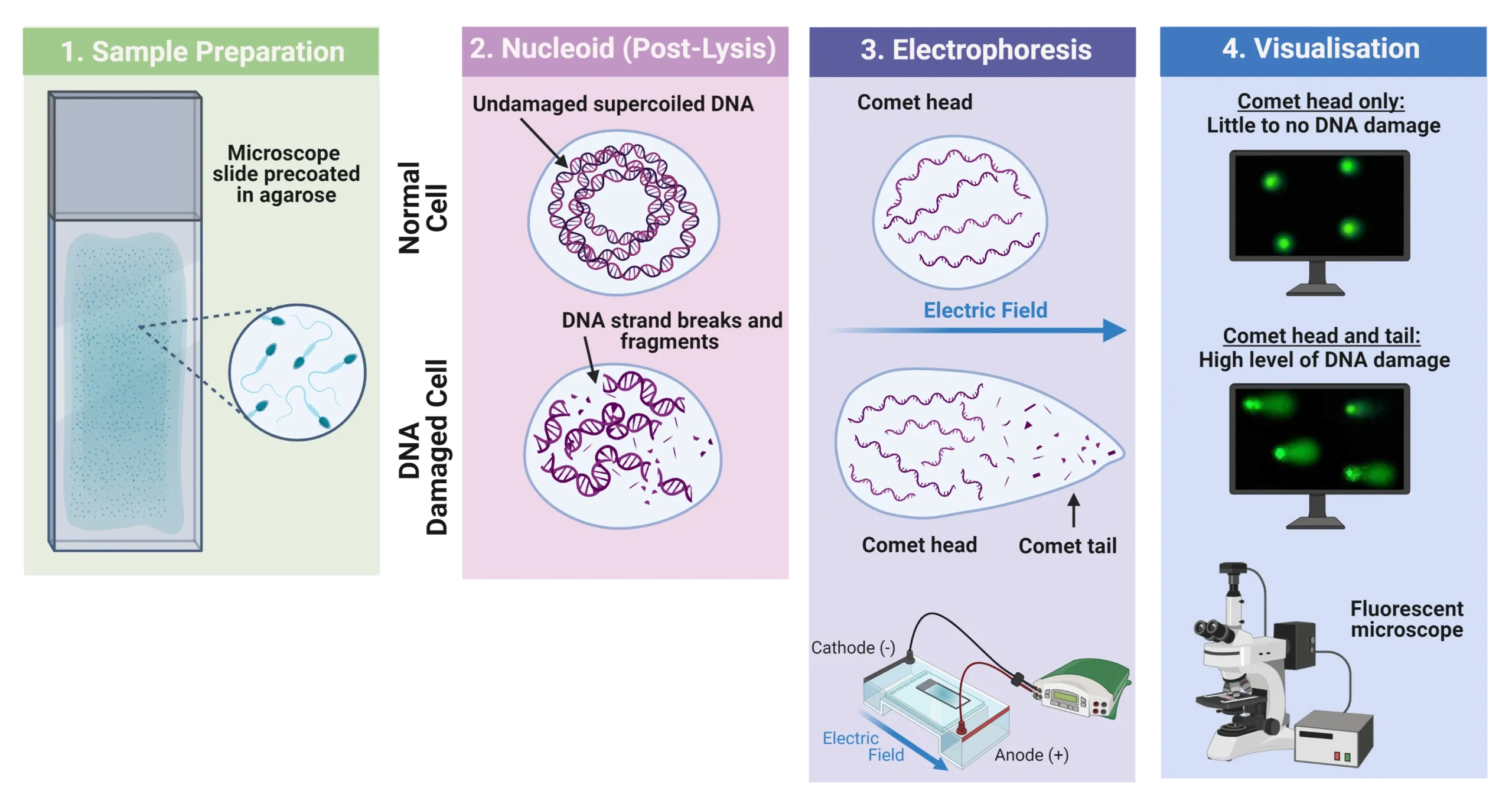
This assay evaluates the potential of a test substance to induce DNA damage by measuring the migration of DNA fragments through an agarose gel under an electric field, forming a characteristic “comet” tail whose length and intensity correlate with the extent of DNA damage. According to ISO 10993-3, the Comet Assay may be applied in both in vitro and in vivo settings. In vitro applications commonly involve cultured mammalian cells, such as human lymphocytes or established cell lines, to assess direct genotoxic effects.
In vivo assessments typically utilize rodent models to evaluate DNA damage in specific organs or tissues following systemic exposure to test materials. The assay’s relevance in biocompatibility testing lies in its capacity to detect primary DNA damage, supporting risk assessment of medical devices that may release genotoxic agents.
The Comet Assay Procedure
I. In Vitro Comet Assay
Purpose
The in vitro Comet Assay is used to assess the genotoxic potential of chemical substances, pharmaceuticals, or environmental agents in cultured cells (e.g., mammalian, human lymphocytes, or established cell lines).
Procedure
Cell Preparation
Cells (e.g., human lymphocytes, CHO, HepG2, or TK6 cell lines) are cultured under appropriate conditions.
Cells are exposed to the test compound for a defined period, usually with and without metabolic activation (S9 mix) to mimic in vivo metabolism.
Cell Harvesting and Embedding
After exposure, cells are embedded in low melting point agarose on a microscope slide pre-coated with normal melting point agarose.
Cell Lysis
Slides are immersed in lysis solution (commonly containing high salt concentration and detergents, such as NaCl, EDTA, Triton X-100, and DMSO) for several hours at 4°C to remove membranes and proteins, leaving nucleoids of supercoiled DNA.
DNA Unwinding
Slides are incubated in alkaline electrophoresis buffer (usually pH >13) for 20–60 minutes to unwind DNA and convert alkali-labile sites into strand breaks.
Electrophoresis
Electrophoresis is conducted under alkaline conditions (typically 25 V, ~300 mA) for 20–30 minutes. Negatively charged DNA fragments migrate towards the anode, forming a “comet tail”.
Neutralization and Staining
Slides are neutralized with Tris buffer (pH ~7.5), dried, and stained using fluorescent DNA dyes (e.g., ethidium bromide or SYBR Green).
Image Analysis
Cells are visualized under a fluorescence microscope, and DNA damage is quantified using image analysis software. Key parameters include:
- Tail length
- % Tail DNA
- Olive Tail Moment (OTM)
Applications
Screening genotoxic agents
Dose-response studies
Mechanistic studies
Evaluation of DNA repair
Advantages
High sensitivity for low levels of DNA damage
Suitable for a wide range of cell types
Requires fewer cells than traditional assays
II. In Vivo Comet Assay
Purpose
The in vivo Comet Assay detects DNA strand breaks in cells directly obtained from animals exposed to a test compound. It is considered a regulatory-relevant assay (e.g., OECD TG 489) for in vivo genotoxicity evaluation.
Procedure
Animal Exposure
Laboratory animals (commonly rats or mice) are exposed to the test compound by oral, inhalation, dermal, or intraperitoneal routes, typically over 1–3 consecutive days.
A vehicle control and a positive control (e.g., ethyl methanesulfonate) are included for validity.
Tissue Collection
After exposure, animals are euthanized, and target organs (e.g., liver, stomach, colon, kidney, lung, or blood) are excised.
Cells are isolated by mechanical or enzymatic dissociation and maintained on ice to prevent DNA degradation.
Cell Embedding and Assay
The same procedures for lysis, alkaline unwinding, electrophoresis, neutralization, staining, and image analysis as in vitro are followed.
Quantification
DNA damage is quantified in a statistically significant number of cells (commonly ≥ 100 cells per animal).
Statistical analysis is conducted to determine treatment-related increases compared to controls.
Applications
Regulatory genotoxicity testing (e.g., OECD TG 489)
Evaluating tissue-specific genotoxicity
Assessing systemic exposure and distribution of genotoxins
Advantages
Reflects whole-body metabolism, distribution, and excretion
Can assess multiple organs/tissues
Useful in combination with other in vivo genotoxicity tests (e.g., micronucleus test)
For more information, click Genotoxicity Testing.
Comparison: In Vitro vs. In Vivo Comet Assay
| Feature | In Vitro | In Vivo |
|---|---|---|
| Test System | Cultured cells | Animal tissues/cells |
| Metabolism | Requires S9 mix | Physiological metabolism |
| Complexity | Simple and controlled | Complex, requires animal use |
| Regulation | Screening or research | Regulatory (OECD TG 489) |
| Tissue Specificity | Single-cell type | Multiple tissues/organs |
| Ethical Consideration | No animal use | Requires animal ethics approval |
The Comet Assay, in both in vitro and in vivo formats, provides a robust, sensitive, and versatile platform for the detection of primary DNA damage such as strand breaks, alkali-labile sites, and incomplete excision repair sites. While the in vitro assay allows controlled mechanistic studies, the in vivo assay offers a physiologically relevant assessment incorporating metabolic activation, systemic distribution, and tissue-specific genotoxicity. Together, they form complementary tools in genetic toxicology and risk assessment.
Certainly! Here’s a rewritten and specialized version of the text tailored specifically for The Comet Assay (single-cell gel electrophoresis):
Why Choose International Nikopharmad Laboratory for the Comet Assay?
ILAC Accreditation and ISO/IEC 17025 Certification
International Nikopharmad Laboratory is fully accredited under ISO/IEC 17025 and recognized by ILAC (International Laboratory Accreditation Cooperation)—affirming our technical competence and strict compliance with internationally accepted quality standards. Our Comet Assay data meet the requirements of global regulatory bodies such as the FDA, EMA, and PMDA, ensuring your submissions are both scientifically robust and regulator-ready.
Specialized Expertise in Comet Assay Implementation
Nikopharmad brings deep expertise in DNA damage assessment through the Comet Assay, a sensitive and versatile method for detecting strand breaks at the single-cell level. Our team of experienced molecular toxicologists and DNA damage experts employs standardized and fully validated protocols in accordance with OECD guidelines and GLP principles. Whether you’re conducting initial screening or confirmatory genotoxicity analysis, we ensure data relevance, reproducibility, and analytical precision.
State-of-the-Art Infrastructure and Technical Rigor
We utilize advanced electrophoresis systems, image analysis software, and high-throughput sample processing platforms to support Comet Assay studies across multiple tissue types and exposure routes. Every assay is performed under strict quality control, with customized assay conditions tailored to your compound’s pharmacokinetic and mechanistic profile.
Competitive Pricing with Scientific Integrity
Our pricing structure is designed to offer cost-effective Comet Assay testing without compromising on quality or regulatory compliance. We help you manage R&D budgets more efficiently while generating data that withstands the highest scientific and regulatory scrutiny.
Rapid Turnaround and Adaptive Project Management
Speed matters in development timelines. Our dedicated project managers and optimized workflows allow for swift initiation, streamlined processing, and fast reporting—without sacrificing data integrity. From protocol design to final reporting, we support agile decision-making and reduce time-to-market.
Confidentiality and Data Protection
We prioritize the security of your intellectual property. All Comet Assay studies are conducted under robust non-disclosure agreements (NDAs), and our data handling systems are built to maintain complete traceability, confidentiality, and integrity at every stage of the project.
To request testing or a complimentary consultation contact Nikopharmad
Partner with Nikopharmad for Expert Comet Assay Testing
When you choose International Nikopharmad Laboratory, you’re choosing a partner with a proven track record in genotoxicity testing—specifically the Comet Assay. Our globally accredited methodologies, scientific depth, operational efficiency, and budget-conscious pricing make us a trusted ally in advancing your product safely through the regulatory landscape.
Trust Nikopharmad to deliver accurate, regulatory-grade Comet Assay data—on time, on budget, and to the highest international standards.