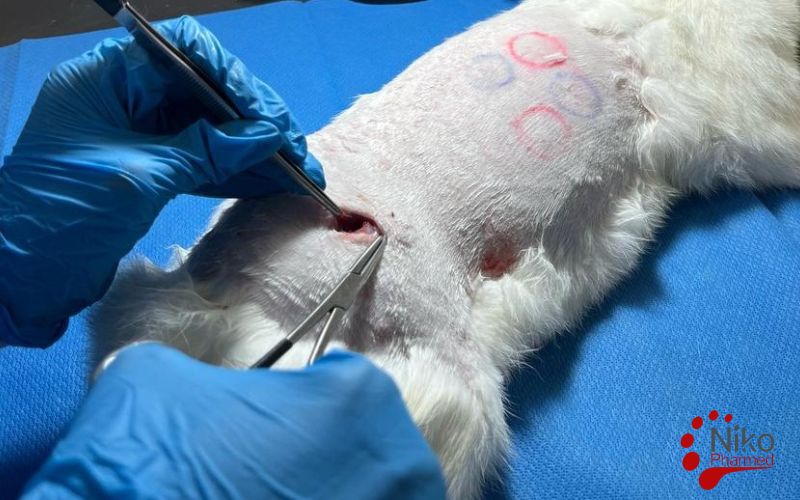
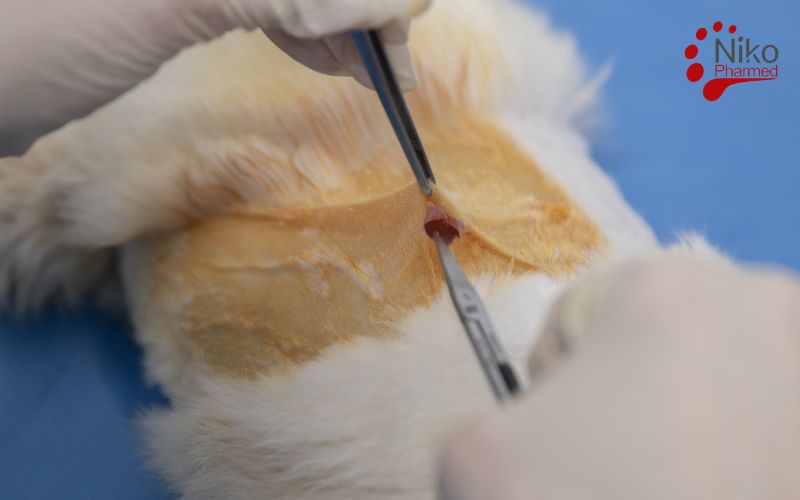
What Is Implantation Testing?
Implantation Testing is a key part of biocompatibility testing for medical devices, evaluating how materials react with living tissue after implantation. Following ISO 10993-6, this test involves placing the material or device into an animal model (usually rabbits) and observing tissue responses like inflammation or fibrosis. The results help determine if the material is safe for long-term use in the human body, ensuring compliance with regulatory standards. This test is crucial for assessing the local tissue compatibility of medical devices before clinical use
With extensive professional experience in preclinical safety evaluation and regulatory compliance, it is clear that implantation testing stands as a foundational tool in the risk assessment of implantable devices. It exemplifies expertise through its scientifically rigorous methodology, authoritativeness through its inclusion in globally recognized standards, and trustworthiness by providing accurate, reproducible data that support patient safety and regulatory approval Implantation Testing is a crucial part of a device’s biological evaluation and is often performed alongside Acute Systemic Toxicity Testing and Hemocompatibility Testing to cover both local and systemic responses to implanted devices.
To request COST-EFFECTIVE Implantation testing contact Nikopharmad
Implantation Testing Procedure (ISO 10993-6)
Study Planning and Ethical Approval
At Nikopharmed Laboratory, all implantation studies are initiated with the development of a comprehensive and meticulously detailed protocol. This includes clear definitions of the test and control items, selection of appropriate implantation sites, justification of animal species, determination of group sizes, designated observation time points, and predefined histopathological evaluation criteria. Every protocol is subjected to rigorous ethical review and must receive formal approval from our Institutional Animal Care and Use Committee (IACUC) or an equivalent ethical oversight body. We ensure full adherence to Good Laboratory Practice (GLP) and uphold the highest standards of animal welfare, reflecting our commitment to scientific integrity, regulatory compliance, and responsible research.
Selection of Animal Model and Implantation Site
ISO 10993-6 recommends choosing an animal species whose tissue response is well-characterized and suitable for the intended clinical application. Common models include rabbits or rats. Implantation sites—typically intramuscular or subcutaneous—are selected based on the clinical use of the device. For instance, an orthopedic material would be evaluated intramuscularly to simulate musculoskeletal interaction.
Preparation of Test and Control Articles
Both test and control materials must be sterilized using a validated method that does not alter their chemical or physical properties. The control—commonly a high-purity polymer such as polyethylene—is essential for baseline comparison. Dimensions, shape, and surface characteristics must be documented, as these affect tissue response.
Surgical Implantation Procedure
Under aseptic conditions and appropriate anesthesia, a surgical incision is made to expose the designated implantation site. Each animal typically receives both test and control implants in a randomized manner to minimize variability. The implants are inserted into a precisely prepared tissue pocket, ensuring minimal trauma and accurate positioning. Wounds are sutured, and postoperative care is provided per veterinary standards.
Post-Implantation Monitoring and Observation Periods
Animals are monitored daily for signs of systemic toxicity, infection, or adverse reactions. ISO 10993-6 recommends multiple time points (e.g., 1 week, 4 weeks, 12 weeks) depending on the device’s expected duration of contact. This ensures both acute and chronic tissue responses are captured.
Euthanasia and Tissue Harvesting
At designated endpoints, animals are humanely euthanized using an approved method. Implantation sites are carefully excised with surrounding tissue intact to avoid artifact generation. Gross pathology observations are recorded during necropsy.
Histopathological Evaluation
Tissue samples are fixed, embedded, sectioned, and stained (typically using Hematoxylin and Eosin). A blinded, board-certified pathologist evaluates cellular responses such as inflammation, fibrosis, neovascularization, necrosis, and foreign body reaction. ISO 10993-6 emphasizes semi-quantitative scoring systems to compare test versus control materials in a statistically valid manner.
Data Interpretation and Reporting
The final report must include comprehensive documentation of procedures, animal health, histopathological findings, statistical analysis, and compliance with ISO requirements. The goal is to determine whether the test article induces an unacceptable local tissue response that may pose a clinical risk.
Implantation Testing Procedure Areas
At Nikopharmed Laboratory, we conduct implantation testing strictly in accordance with ISO 10993-6:2021, ensuring precise evaluation of the local biological effects of medical devices and materials within appropriate anatomical environments. The standard specifies various implantation sites that reflect the intended clinical use of the device. Our laboratory is equipped and experienced in performing implantations in the following anatomical regions, each selected based on the material’s functional application:
Subcutaneous Tissue
This site is commonly used for general biocompatibility screening of materials with indirect or surface contact. It allows clear evaluation of tissue reaction such as inflammation, fibrosis, and foreign body response. Subcutaneous implantation is minimally invasive, making it suitable for early-phase material evaluation.
Muscle Tissue
Intramuscular implantation provides more dynamic tissue interaction, particularly for materials intended for orthopedic or soft-tissue interfacing. This method is more sensitive to mechanical irritation and deeper inflammatory responses, offering a more rigorous test environment for evaluating local toxicity or chronic irritation potential.
Bone Tissue
For materials intended for load-bearing or orthopedic use (e.g., screws, bone fillers, or implants), direct implantation into bone is essential. This model allows us to assess osseointegration, bone remodeling, and any adverse interactions with bone marrow or cortical structures. We use advanced imaging and histology to analyze bone-implant interface characteristics.
Brain Tissue
In specialized studies involving neuro-devices or brain-contacting biomaterials, we perform implantations into brain tissue using highly controlled neurosurgical techniques. This model is used to evaluate neuroinflammatory responses, glial activation, and tissue necrosis, which are critical for ensuring the safety of central nervous system (CNS) applications.
Our team of in vivo specialists applies rigorous surgical protocols, GLP-compliant data recording, and standardized histopathological scoring systems to ensure scientific integrity and regulatory-grade reliability. Each anatomical model is selected based on risk assessment and intended clinical application, reinforcing our commitment to experience-driven, expert-guided, and trustworthy preclinical evaluation. As a laboratory that upholds E-E-A-T principles, we ensure that every implantation study generates data that is clinically relevant, ethically obtained, and scientifically robust, supporting safe and effective medical innovation.
The in vivo approach used in Implantation Testing can be combined with Cytotoxicity Testing and Skin Sensitization Testing to identify potential biological reactions before full-scale clinical trials
To request COST-EFFECTIVE Implantation testing contact Nikopharmad
Implantation Testing Lab
At International & Accredited Lab Nikopharmad Laboratory Network, we are committed to providing accurate, reliable, and high-quality implantation testing services for medical devices and pharmaceutical products. Holding the esteemed ISO 17025 certification and ILAC accreditation, we adhere to internationally recognized standards, ensuring that our testing processes meet the highest quality and regulatory requirements.
Why Choose Nikopharmad Laboratory for Implantation test?
ILAC accreditation
Nikopharmad Laboratory is proudly certified with ISO 17025 and holds ILAC accreditation. These prestigious certifications ensure that our laboratory operates with the highest standards of quality and competence, providing reliable and internationally recognized testing services for medical devices and pharmaceutical products. Our commitment to maintaining these certifications demonstrates our dedication to excellence and compliance with global regulatory requirements.
Expertise and Experience
With extensive experience in the medical and pharmaceutical sectors, our laboratory is equipped with cutting-edge technology and a team of highly trained professionals who ensure accurate, timely, and thorough testing results.
Efficient and Timely Results
We understand the importance of time in product development. Our laboratory strives to deliver fast and reliable results, ensuring your products are tested and ready for market entry as efficiently as possible.
Confidentiality and Integrity
At Nikopharmad, we prioritize the confidentiality of your sensitive data and intellectual property. Our strict adherence to confidentiality agreements guarantees that your information remains protected throughout the testing process.
Global Compliance and Support
Whether you are preparing to enter the FDA, or other global markets, our laboratory ensures that your products comply with all necessary regulatory standards, facilitating a smooth path to market approval.
Choose Nikopharmad for Your Implantation Testing Needs
By choosing International Nikopharmad Laboratory, you are selecting a trusted partner that not only meets but exceeds international testing standards. Our ISO 17025 certification, expert team, and commitment to excellence make us the ideal choice for ensuring the safety, compliance, and success of your medical devices and pharmaceutical products.
Frequently Asked Questions About Implantation Testing
1. What is Implantation Testing?
Implantation Testing is a procedure used to assess the local tissue response to a medical device or material after it has been implanted in an animal model. The test helps determine whether the material causes any harmful reactions, such as inflammation, necrosis, or fibrosis, and ensures that it is safe for human use.
2. Why is Implantation Testing important for medical devices?
Implantation testing is crucial because it evaluates the biocompatibility of medical devices intended for long-term or permanent implantation in the human body. This test ensures that materials do not cause adverse local reactions, which could lead to complications when used in patients.
3. What animals are used in Implantation Testing?
Typically, rabbits are used for implantation testing due to their well-characterized tissue response. However, other animal models may also be used depending on the device and testing requirements.
4. How is Implantation Testing conducted?
During implantation testing, the test material is surgically implanted into the tissue of an animal model, usually in the subcutaneous or muscle tissue. The animal is then observed for signs of irritation, inflammation, or other tissue reactions over a predefined period, and histopathological analysis is performed to evaluate the results.
5. What are the common observation periods for Implantation Testing?
Implantation testing typically includes several observation time points, such as 1 week, 4 weeks, or 12 weeks, depending on the device’s expected duration of contact with tissue. These time frames help capture both acute and chronic tissue responses.
6. How is the success of Implantation Testing measured?
The success of implantation testing is measured by evaluating the tissue response at the implantation site. If significant inflammation or necrosis occurs, the material may be considered non-biocompatible. Scoring is typically done through histopathological analysis and tissue samples.
7. What standards are followed for Implantation Testing?
ISO 10993-6 provides the guidelines and requirements for conducting implantation testing for medical devices. This international standard ensures that testing is done in a scientifically rigorous and ethically responsible manner.
8. How does Implantation Testing help with regulatory approval?
Implantation testing is a crucial part of the biocompatibility assessment required for medical devices to obtain regulatory approval. It helps demonstrate that the device or material is safe for use in humans and meets the necessary safety standards set by health authorities like the FDA and EMA.