What is Irritation Testing?
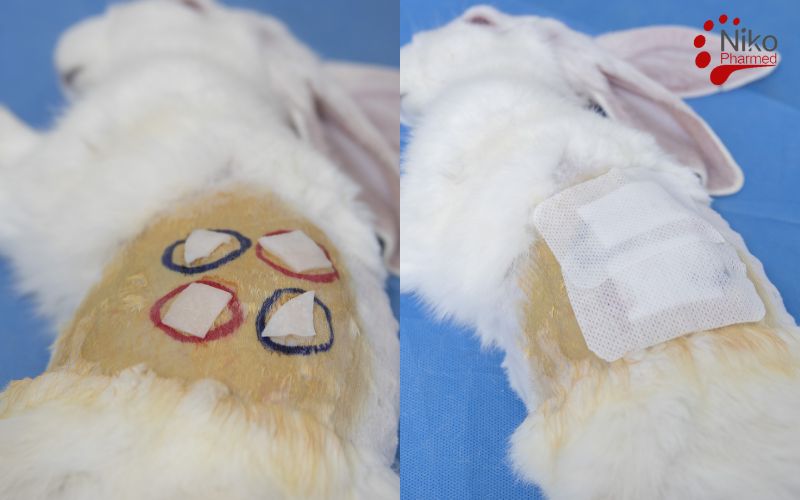
The irritation test, as defined in ISO 10993-10, is a biological evaluation used to assess the potential of materials or medical devices to cause irritation upon contact with skin or mucosal surfaces. This test is designed to identify any inflammatory reactions, such as redness (erythema) or swelling (edema), that may occur due to exposure to the material. The irritation test is typically conducted using animal models, often rabbits, where the material is applied to the skin or mucosa, and the reactions are observed over a specified period. These observations are then scored based on the severity of the irritation, with scores ranging from 0 (no irritation) to 4 (extreme irritation).
The results help classify the material as either non-irritant or irritant, guiding the safety and suitability of the material for medical device applications. This test is essential for ensuring the biocompatibility of medical devices in compliance with the ISO 10993-10 standard, which ensures that devices are safe for patient use.
Irritation testing is an integral part of a comprehensive biocompatibility evaluation and is routinely performed alongside Cytotoxicity Testing and Sensitization Testing to ensure device safety for intact skin and mucosal contact.
To request testing or a complimentary consultation contact Nikopharmad
Irritation Testing Areas
- Skin
- Intradermal
- Chronic Skin (Repeated Exposure)
- Penile
- Vaginal
- Acute Ocular
- Chronic Ocular
Irritation Testing Procedure
Material Selection
Select the material or device that is to be tested. It should be in the form intended for clinical use, ensuring that it accurately represents the final product.
The material can be a solid, liquid, or gel, depending on the type of device being tested.
Preparation of Test Animals
The test is typically conducted using rabbits, although other animal models may be used if specified.
The animals should be healthy and acclimatized to the laboratory environment prior to testing.
The test site (usually the skin or mucosa) is shaved and cleaned to remove any contaminants or hair that could interfere with the results.
Application of the Test Material
The test material is applied to the skin or mucosal surface. The method of application may vary (e.g., as a patch, liquid, or gel) and should follow the guidelines in the standard.
The duration of exposure should be standardized, typically ranging from a few hours to several days, depending on the test protocol.
The material should be applied in a controlled manner to ensure even distribution and appropriate contact with the test surface.
Observation of Reactions
After the exposure period, the test animals are observed for signs of irritation, such as erythema (redness), edema (swelling), or other inflammatory responses.
The observations should occur at specific time intervals post-application, typically at 24, 48, and 72 hours, and may continue for up to a week in some cases.
Scoring of Irritation
The observed irritation responses are scored based on severity using a standardized scale, usually from 0 to 4, where:
-
-
- 0 = No irritation (no redness or swelling).
- 1 = Mild irritation (slight redness or swelling).
- 2 = Moderate irritation (clear erythema or edema).
- 3 = Severe irritation (pronounced redness or swelling).
- 4 = Extreme irritation (necrosis or ulceration).
-
This scoring system helps quantify the degree of irritation for each test animal.
Data Analysis and Interpretation
The individual scores from different test animals are analyzed. If significant irritation is observed in any of the test animals, the material may be classified as an irritant.
Based on the cumulative results, the material is classified as either:
-
-
- Non-irritant: If no or only mild irritation occurs.
- Irritant: If moderate or severe irritation is observed.
-
If necessary, additional tests or modifications to the material may be considered based on the findings.
Reporting of Results
The results of the test, including the observed reactions, scores, and conclusions, should be documented and reported in compliance with the standard.
Any recommendations for modifications to the material or further testing should be included based on the findings.
The irritation test according to ISO 10993-10 is essential for ensuring that materials used in medical devices do not cause adverse local reactions when in contact with the skin or mucosal surfaces. By following this structured procedure, manufacturers can assess the biocompatibility of materials and confirm their safety for clinical use.
In addition to Irritation Testing, other essential biocompatibility tests include Pyrogenicity Testing and Hemocompatibility Testing which can be done slongside this test.
Irritation Testing Lab
At International & Accredited Lab Nikopharmad Laboratory Network, we are committed to providing accurate, reliable, and high-quality biocompatibility testing services for medical devices and pharmaceutical products. Holding the esteemed ISO 17025 certification and ILAC accreditation, we adhere to internationally recognized standards, ensuring that our testing processes meet the highest quality and regulatory requirements.
Why Choose Nikopharmad Laboratory?
ILAC accreditation
Nikopharmad Laboratory is proudly certified with ISO 17025 and holds ILAC accreditation. These prestigious certifications ensure that our laboratory operates with the highest standards of quality and competence, providing reliable and internationally recognized testing services for medical devices and pharmaceutical products. Our commitment to maintaining these certifications demonstrates our dedication to excellence and compliance with global regulatory requirements.
Comprehensive Testing Capabilities
We offer a broad range of biocompatibility tests , each designed to assess the safety and performance of your products in compliance with international regulatory frameworks.
Expertise and Experience
With extensive experience in the medical and pharmaceutical sectors, our laboratory is equipped with cutting-edge technology and a team of highly trained professionals who ensure accurate, timely, and thorough testing results.
Efficient and Timely Results
We understand the importance of time in product development. Our laboratory strives to deliver fast and reliable results, ensuring your products are tested and ready for market entry as efficiently as possible.
Confidentiality and Integrity
At Nikopharmad, we prioritize the confidentiality of your sensitive data and intellectual property. Our strict adherence to confidentiality agreements guarantees that your information remains protected throughout the testing process.
Global Compliance and Support
Whether you are preparing to enter the FDA, or other global markets, our laboratory ensures that your products comply with all necessary regulatory standards, facilitating a smooth path to market approval.
Choose Nikopharmad for Your Irritation Testing Needs
By choosing International Nikopharmad Laboratory, you are selecting a trusted partner that not only meets but exceeds international testing standards. Our ISO 17025 certification, expert team, and commitment to excellence make us the ideal choice for ensuring the safety, compliance, and success of your medical devices and pharmaceutical products.
Reference:iso.org