What is Ph Testing?
pH testing is a fundamental analytical procedure used in chemistry to determine the acidity or alkalinity of an aqueous solution. It quantifies the concentration of hydrogen ions (H⁺) present, expressed on a logarithmic scale ranging from 0 to 14, where a pH of 7 is neutral, values below 7 indicate acidity, and values above 7 indicate alkalinity. The standard pH measurement is typically performed using a calibrated pH meter or pH indicator strips, which provide a precise and reliable assessment essential for various scientific, industrial, and environmental applications. This test is often paired with carbohydrate analysis to determine the complete chemical profile of the sample.
Different Types of PH Testing
1. pH Indicators
Ph indicators are chemicals that change color depending on the pH of the solution. These compounds are usually weak acids or bases, which exhibit different colors at different pH levels. The color change is due to protonation or deprotonation of the indicator molecule as the pH of the solution changes.
Mechanism:
When an indicator is in a solution, it exists in a dissociated and undissociated form, which have different colors.
The equilibrium between these forms shifts depending on the concentration of hydrogen ions ([H+][H^+]) in the solution (which is related to pH).
Common pH Indicators and Their pH Ranges:
Phenolphthalein: Colorless in acidic solutions (pH < 7) and pink in basic solutions (pH > 7).
Methyl Orange: Red in strongly acidic solutions (pH < 3.4), orange in weakly acidic (pH 3.4-4.4), and yellow in basic solutions (pH > 6).
Bromothymol Blue: Yellow in acidic (pH < 6), green in neutral (pH ≈ 7), and blue in basic (pH > 7).
Advantages:
Simple, quick, and inexpensive.
No special equipment is required, just the indicator and the solution.
Limitations:
Provides qualitative information (rough pH range), not quantitative values.
The color transition may be subjective, especially for indicators with narrow pH ranges.
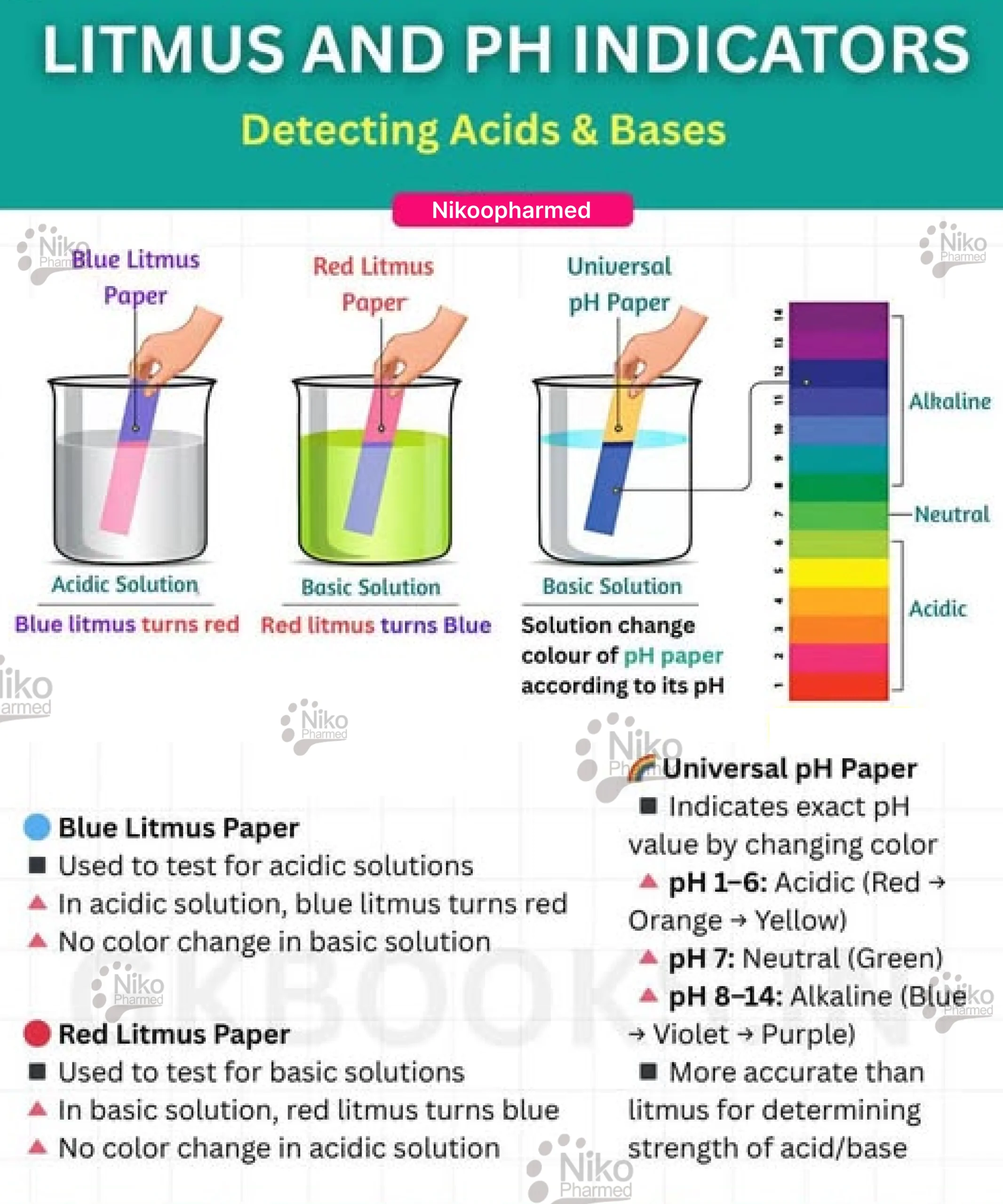
2. pH Strips (Litmus Paper)
Ph strips, also called litmus paper, contain pH-sensitive dyes and work similarly to pH indicators. The paper changes color depending on the solution’s pH when dipped into it(visible in the ph testing image below) ph testing
Mechanism:
The pH indicator in the strip reacts with the solution’s hydrogen ion concentration, causing the strip to change color.
The color is then compared to a standardized color chart that corresponds to different pH values.
Advantages:
Quick and easy to use for rough estimates of pH
Often available in both universal pH strips (that cover a wide pH range) and specific litmus strips (for testing acidic or basic solutions).
Limitations:
Like pH indicators, they only provide qualitative results, meaning a general range (for example, 4-6 or 7-9), not an exact pH value.
Less precise than a pH meter.
3. Titration
Titration is a quantitative method used to determine the exact concentration of an acid or base in a solution by adding a reagent (called the titrant) of known concentration until the reaction reaches the equivalence point, where the amount of acid equals the amount of base.
Mechanism:
The titrant (e.g., NaOH) is added to the solution (e.g., HCl) slowly and the pH is monitored.
The equivalence point is reached when the amount of acid and base are stoichiometrically equal.
The equivalence point can be detected using either a pH indicator or a pH meter.
Steps:
- A known volume of the acid is placed in a flask.
- A pH indicator is added (if not using a pH meter).
- The titrant is added drop by drop, with constant stirring.
- The endpoint (or equivalence point) is identified by a color change or pH shift.
- Using the volume of titrant used, the concentration of the unknown acid (or base) can be calculated using the formula:
M1V1=M2V2
where:
- M1 and M2 are the molar concentrations of the acid and base.
- V1 and V2 are their respective volumes.
Types of Titrations:
- Strong Acid vs. Strong Base Titration: The equivalence point occurs at pH 7.
- Weak Acid vs. Strong Base Titration: The equivalence point occurs at pH > 7.
- Strong Acid vs. Weak Base Titration: The equivalence point occurs at pH < 7.
Advantages:
Highly accurate and precise for determining the concentration of acids or bases.
Can be applied to various types of acids and bases.
Limitations:
Requires careful measurement and appropriate choice of indicator (or a pH meter).
Can be time-consuming for complex reactions or weak acid/base titrations.
4. Volatile Acidity (VA)
Volatile acidity refers to acids that can vaporize under standard conditions in ph testing, primarily acetic acid (CH₃COOH) and other short-chain fatty acids, which are commonly found in vinegar, wine, and other fermented products. Volatile acidity testing measures the amount of these acids in a liquid sample by distillation.
Mechanism:
In the distillation method, the sample is heated to release the volatile acids into the vapor phase. The vapor is then condensed and collected.
The acidity of the collected distillate is then measured, usually by titration with a standard base (e.g., sodium hydroxide, NaOH) to determine the concentration of acetic acid (or other volatile acids).
The result is often expressed as the amount of acetic acid (g/L) or grams per 100 mL of the sample.
Steps:
- The sample is heated, and the volatile acids are distilled off.
- The distillate is collected and titrated with a standard base (e.g., NaOH).
- The amount of titrant used gives the concentration of volatile acids in the original sample.
Advantages:
Essential in the food industry (wine, vinegar production) and other fermenting processes.
Useful for monitoring the quality of fermented products, where high volatile acidity can indicate spoilage or over-fermentation.
Limitations:
Does not measure non-volatile acids (e.g., citric acid, tartaric acid).
Requires specialized distillation equipment.
Comparison of Methods
| Method | Type of Data | Advantages | Limitations |
|---|---|---|---|
| pH Indicators | Qualitative (range) | Simple, quick, inexpensive | Rough estimates, subjective color change |
| pH Strips | Qualitative (range) | Convenient, portable, easy to use | Rough estimates, limited precision |
| Titration | Quantitative | Highly accurate, precise, suitable for any acid/base | Requires careful technique, slower process |
| Volatile Acidity | Quantitative | Essential for fermentation monitoring, specific for volatile acids | Requires distillation, specialized equipment |
pH indicators and pH strips are quick and simple methods but only provide qualitative pH ranges.
Titration is a quantitative method for determining the exact concentration of acids and bases and is highly accurate, but requires more care and time.
Volatile acidity testing is crucial for food and beverage industries, particularly for monitoring fermentation processes and ensuring quality.
Each method has its specific use case, and the choice depends on the required accuracy, type of analysis, and available equipment.
Why Choose Nikopharmad for Your Ph Testing Needs?
Accredited & Certified
Nikopharmad is ISO/IEC 17025 certified and ILAC-accredited, ensuring our pH testing meets international standards for accuracy, reliability, and regulatory compliance.
Validated Methods
We follow globally recognized methodologies, including USP <791>, EP 2.2.3, ICH Q6A, and ISO standards. Our protocols use calibrated pH meters, standardized buffers, and temperature compensation for precise ph scale.
Expert Staff & Advanced Equipment
Our team of analytical chemists and QA specialists operates advanced instrumentation suitable for a wide range of sample types—from pharmaceuticals to cosmetics and water.
Fast Turnaround & Flexible Services
With optimized workflows, we offer rapid and reliable results to support product development, quality control, and regulatory submissions. Custom testing plans and volume discounts are available.
Data Integrity & Regulatory Readiness
All results are recorded in 21 CFR Part 11-compliant systems, ensuring data security, audit readiness, and full traceability. Reports are formatted for global regulatory acceptance.